APPARATUS REQUIRED:
i) Test tube
ii) Conical flask
iii) Beaker
iv) Funnel
CHEMICALS REQUIRED:
i) Sample (S3)
ii) AgNO3
iii) dil.H2SO4
iv) Conc.H2SO4
v) BaCl2
THEORY:
Qualitative analysis concerns the finding out the Constituent present in the substance. The basis of Qualitative inorganic analysis is the properties of ions present in the salt. Analysis is done in dry and wet ways.
i) Dry test: In dry test characteristic properties of a substance when subjected to the acids,alkalis, oxidizing and reducing agents generally with references to its Colour, odour and state are the basis of analysis. Dry test is always performed before a wet test.
ii) Wet test: In wet tests radicals are detected in solution by their ionic reactions. Acid radicals are identified by their reaction with the particular reagent metal ions are classified into dermite analysis group.
PHYSICAL PROPERTIES:
Sample: S3
Colour: Greenish white
State: Crystalline solid
Solubility: Soluble in water
Odour: Characteristic odour.
A) Dry test for Acid Radicals:
| Experiment | Observation | Inferences |
| i) Action of dil. H2SO4: 0.2 gm of substance in a test tube was treated with 2-3 ml of dil H2SO4 and warmed gently. | Coloured gas with pungent smell was observed and no effervescence took place. | Absence of CO2–, SO2– , S—, NO2– |
| ii) Action of conc.H2SO4: 0.2g of substance in a test tube was treated with conc.H2SO4 and warmed gently. | Pungent acid turns evolved often coloured brown by NO2 and Coloured in addition. of copper chips. | Presence of NO3– |
Reaction Involved:
NaNO3 + H2SO4 ————-> NaHSO4 + HNO3
2HNO3 ————————> H2O + 2NO + 3O
3Cu + 3O ———————> 3Cuo
3CuO + 6HNO3 ————-> 3Cu(NO3)2 + 3H2O
3NaNO3 + 8H2SO4 + 8Cu + O2 ———-> 8NaHSO4 + 8Cu(NO3)2 + 4H2O + 2NO2
B) Wet test for Acid Radicals:
Wet test are carried out with the solution of salt:
| Experiment | Observation | Inferences |
| i) AgNO3 Test: 1 ml salt solution was taken in a test tube and 1 ml of dil.HNO3 then a few drops of AgNO3 solution was added. | White ppt was observed but didn’t dissolve in NH3. | Absence ofCl–, Br–, and I–. |
| ii) BaCl2 Test: 1 ml of salt solution was taken in the test tube and BaCl2 solution was added. | White ppt was observed and Insoluble in dll.HNO3 | Presence of SO4—. |
| iii) Test for Nitrate: 2ml of salt solution was taken in the test tube, 2 ml of conc. H2SO4 was added slowly cooled and freshly prepared FeSO4 Solution was poured through the wall of the tube keeping it in an inclined position. | Brown ring was formed in the contactof two solutions. | Presence of NO3– . |
Reaction of SO4—:
Na2SO4 + BaCl2 ———> BaSO4 + 2NaCl
Reaction of NO3—:
NaNO3 + H2SO4 ———> NaHSO4 + HNO3
6FeSO4 + 2HNO3 + 3H2SO4 ——–> 3Fe(SO4)3 + 2NO + 4H2O
FeSO4 + NO ————–> [FeCNO]SO4
C) Test for Basic RadicalS:
Dry test for basic radicals:
| Experiment | Observation | Inferences |
| 0.29 of the substancewas heated in a dry test tube and change that took place was observed. | i) Sound was not produced. | i) Absence of NaCl, Pb(NO3)2. |
| ii) Fusible residue was observed. | ii) Maybe Mg or K salt. | |
| iii) The color was not changed. | iii) Absence of Zn, Pb,Mn, CO. | |
| iv) On strong heating the color changed from blue to black. | iv) May be Cu salt. | |
| v) Ammonia gas evolved turning moist red litmus to blue. | v) May be ammonium salt. |
D) Wet Test for Basic Radical:
Wet test is carried out with the solution of substance 0.5 g of the given salt mixture was dissolved in suitable solvent and diluted to about 15 ml which was used for basic radical separation.
Group separation of cation:
Dil.HCl was added drop by drop to the salt solution, but ppt was not formed.
| Absence of group (I) metals: | HCl concentration was adjusted to 0.3 N Heated to boiling and H2S gas was passed. Black ppt was formed.. |
| Presence of group (II) metals: | The solution was boiled to remove H2S 1-2 ml of conc. HNO3 was added in boiled 1-2g of NH4Cl and dil NH3 was added till alkaline and then 1 ml in excess and boiled for 1 min. No.ppt was formed. |
| Absence of group (III A) metals: | 2-3 ml of dil NH3 was added and heated then H2S gas was passed. No ppt was formed. |
| Absence of group (III B) metals: | H2S was boiled off and concentrated to half volume 0.2g of NH4Cl was added, made alkaline with few drops of conc.AlH3 and (NH4)2CO2 added with stirring in slight excess kept. Kept in a water bath at 50-60°C for 5 min. No ppt was observed. |
| Absence of group (IV) metals: | Na2HSO4 was added 2 ml of conc.HNO3 was added evaporated continuously to dryness until no shite fumes white residue forms presence of group V metals. |
Confirmatory test for group (II) metalion:
The ppt was transferred into the porcelain basin 5 ml of dil HNO3 was added, boiled for a few minutes and then the solution was washed with filter paper and water. There was no residue and filtrate was a bluish green presence of Co++ . The filtrate was then treated with dil.H2SO4 and NH3, no charge was observed, so it was heated with acetic acid and ferricyanide solution where reddish brown ppt. was formed. Hence the presence of Cu++ was confirmed.
Confirmatory test for group (III B) metalion:
The ppt was transferred into a small beaker then dil.HCl was added, Stirred and filtered. Then in filtrate excess of NaOH was added then 1 ml of 3% H2CO solution was added after removing H2S gas. Then again after cooling and adding NaOH and H2O2 it was boiled and filtered again. The filtrate may contain stat Na₂ (ZnO2). So it was divided into two parts. The one part was acidified with dil.acetic acid and H2S was passed whereas another part was acidified with acetic and potassium ferrocyanide was added where in both cases with ppt. was observed. Hence the presence of Zn++ was confirmed.
Reaction Involved:
A) For Acid Radical
a) wet ways
i) NaCl + H2SO4 ————-> Na2SO4 + HCl ↑
2Na x + H2SO4 ————> Na2SO4 + 2x ↑
ii) NaNO3 + H2SO4 ———-> NaHSO4 + HNO3
2HNO3———————> H2O + 2NO + 3O
3Cu + 3O —————-> 3CuO
3CuO + 6HNO3——–> 3Cu(NO3)2 + 3H2O
8NaNO3 + 8H2SO4 + 3Cu + O2———-> 8NaHSO4 + 3Cu(NO3)2 + 4H2O + 2NO2
b) Wet ways
i) AgNO3 + Cl – ———> AgCl + NO–3
AgCl + 2NH4OH ——> [Ag(NH3)2]Cl + H2O
[Ag(NH3)2]Cl + HNO3 ——-> AgCl ↓ + NH4NO3
ii) NaNO3 + H2SO4 ——–> NaHSO4 + HNO3
6FeSO4 + 2HNO3 + 3H2SO4 ———> 3Fe2(SO4)3 + 2NO + 4H2O
FeSO4 + NO ———-> [Fe(NO)]SO4
B) For Basic Radicals:
- Dry test:
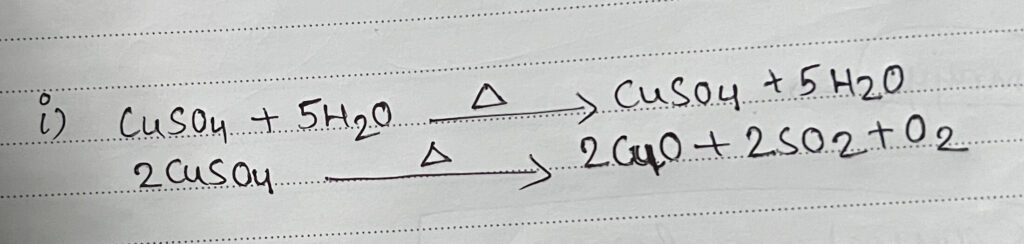
RESULT:
Hence, by the complete analysis of given mixture, the acidic radical present on mixture were Cl– and SO4— and basic radical were Cu++ and Mg++ respectively.
CONCLUSION:
In this way, by the complete analysis of salt, we can detect the acidic and basic radicals respectively.
PRECAUTION:
– Apparatus should be handled carefully.
– Chemicals should be added in the proper amount.
– Boiling and cooling should be done carefully.
– All the tests should be done carefully and properly.
