PHYSICAL PROPERTIES:
Colour: Brownish yellow.
State: Amorphous solid
Solubility: Soluble in water
Odour: Odourless:
THEORY:
Qualitative analysis is concerned with the finding out the constituent present in a substance. The basis of qualitative inorganic analysis is the property of ionic present in a salt analysis is done in two ways. They are dry and wet test:
i) Dry Test: In dry test characteristic properties of a substance when subjected to the acids, alkali, oxidizing and reducing agent generally with difference to its colour odour and state are basic of analysis. Dry test is performed before the wet test.
ii) Wet Test : In wet tests radicals are soluble to their radicals. Acid radicals are identified by the reaction with the particular reagent metal ion and are classified into various. analysis of group basic for the classification of sulphide, hydroxides and carbonate i.e various media and their solubility product.
A: Acids Radicals:
1) Dry test for Acid Radicals:
i) Action of dil.HCl:
| Experiment | Observation | Inferences |
| 0.8 gram of given salt is treated with 2-5 ml of dil.HCl in the test tube. | Effervescence takes place and colourless, odourless gas evolves which farms lime water milky. | CO22 gas from carbonate or bicarbonate. |
ii) Action of Conc.H2SO4:
| Experiment | Observation | Inferences |
| About 0.2 grams of given salt is treated with 2-3 drops of ml of conc.H2SO4 in a test tube . | Colourless gas evolved with pungent odour which fumes in air and produces white fumes with a glass rod moistened with conc.NH3 solution. | HCl gas from chloride. |
Reaction Involving dry test for Acid Radicals:
a) Test with dil.HCl
Carbonate and bicarbonate liberate CO2 as a Colourless and Odourless gas.
CaCO3 + 2HCl ————-> CaCl2 + CO2 + H2O
2NaHCO3 + 2HCl ————> 2NaCl + 2CO2 + 2H2O
MgCO3 + 2HCl —————-> MgCl2 + CO2 + H2O
b) Test with conc.H2SO4
Halides liberates HX CX = Cl, Br, I as colourless and pungent gas.

2) Wet test for Acid Radicals:
| Experiment | Observation | Inferences |
| i) AgNO3 Test: 1 ml of salt solution in a test tube. Added 1 ml of dil.HNO3 and then a few drops of AgNO3 was added. | i) White ppt.dissolved in NH3 solution and reappears when acidified by dil.HNO3. | i) Presence of Cl– ion. |
| ii) BaCl2 Test: 1ml of salt solution in the test tube and BaCl2 solution was added. | ii) White ppt. Dissolved in dil.HNO3 giving effervescence with no smell. | ii) Presence of CO3—ion. |
Reaction Involving wet and confirmatory test of Acid radicals:
a) Reaction of Cl– ion:
i) With the AgNO3 solution.
FeCl3 + AgNO3 ————-> AgcL + fe[(NO3)2]
white curdy ppt. of AgCl soluble in dil HNO3 but soluble in dil. NH3 solution.
b) Reaction of CO3—:
i) With BaCl2 solution.
White ppt. of barium carbonate soluble in minerals acids.
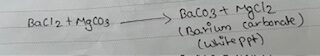
B) Basic Radicals:
1) Dry test for Basic Radicals:
| Experiment | Observation | Inference |
| A small amount ofgiven salt is heatedin a dry test tube. | i) White infusible residue left. | Maybe the presence of Mg salt. |
| ii) Colour changed into a black colour. | ii) May be the presence of Fe salts. |
2) Wet test for Basic Radicals:
a) Preparation of solution:
About 19 m of salt mixture (S2) was dissolved in. suitable solvent in water in a 100 ml beaker and the solution was boiled for 10 minutes and after boiling the solution was filtered with the help of filter paper and funnel. And the clear prepared solution was taken for systematic group separation of cations.
b) Systematic group separation:
To the prepared solution of the beaker a few drops of HCl were added No ppt the prepared solution proceeded further as filtrate.
| Residus: may be PbCl2, HgCl2, AgCl2 white absence of group (I) | Filtrate adjusts HCl conc. to 0.3 N Heat to boiling and passes H2S to a small portion of the solution if ppt forms pass H2S or the complete precipitation. |
| Residue: May be HgS black, PbS black, B2S3 black, SnS2 yellow, CuS black,SbeS3 orange, AS2S3 yellow absence of group (II) | Filtrate: Boil the solution to removeH2S, add 2-2 ml of conc.HNO3 and boil, add 1-2gm of NH4CL and dil.NH3 and then 1 ml in excess boil for 1 min. |
| Residue: May be Fe(OH)3 reddish brown present , Cr(OH)3 green, Al(OH)3 white, MnO2H20 brown group (III) A present: | Filtrate: Added 2-3 ml of dil.NH3 and heat. To a small portion pass H2S if ppt forms, pass H2S to the whole solution. |
| Residue: Group (III B) absence. | Filtrate: This must not be given further ppt.with H2S boil off H2S and concentrate to half of volume and 0.259gm NH4Cl alkaline with a few drops of conc. NH2 and then add (NH4)2CO3 solution with stiminy in sunlight. |
| Residue: Group (IV) absence | Filtrate: May contain Mg++ , Na+ salt. A small portion of solution add Na2HPO4 white ppt indicates Mg++ group V present. |
Analysis of Group IIIA:
The ppt may contain Fe(OH)3, AI(OH)3 Cr(OH)3 and little MnO2. XH2O pieces a hole in the filter paper and transfers the ppt into a small beaker. with a small volume of water Add 1 to 10 mg of solid Na2O2. Add a few ml of NaOH Solution Bell gently until evolution of or ceases. Filter and wash with a little hot water.
| Residue: Dissolve a portion of the ppt in dil.HCl filter if necessary divided into two parts. | Filtrate: May contain Na2CrO4 yellow or NaAIO2 (colourless) if colourless, Cr is absent and need not be considered further if the solution is yellow, Cr is indicated to divide the solution in three parts. |
| i) To one portion add NH4SCN solution .No deep red colouration forms Fe++ absent. | i) With acetic acid and lead acetate no yellow ppt Cr absent. |
| ii) To another portion, added potassium ferrocyanide solution blue colouration or ppt forms Fe++ present. | ii) With dil. HNO3,cool and add alcohol and H2O2 shake blue upper ppt not seen or absent. |
| iii) Mn absent. | iii) With dil.HCl and dil. NH3 is not white pet. Al absent. |
Reaction Involve:
Intense blue ppt of potassium ferric cyanide insoluble in dil.HCl but soluble in Conc. HCl and in oxalic acid decompose by caustic Alkali:
FeCl3 + K[Fe(CN)6]———–> K Fe[Fe(CN)6]3KCl
Analysis of Group (V):
80% of dry residue of group ‘v’ with 3-1 ml of water Stir and warm for 1 minute and filter of completely dissolvent, dilute, somewhat and divide into 3 parts i), ii) and iii) for test of Mg++, Na+, k+ respectively.
| Residue: It residue, dissolve in dil HCl, divide into two parts . | Filtrate: Divide into two parts. |
| i) 1st part with acetic Acid and ammonia solution and warm to dissolve any ppt. to xine add little NH2Cl Solution to the test solution. Followed by the ammonical axine reagent and teat to boiling point for 1-2 min, yellow ppt of myoxinate forms Mg++ present, | i) Add a little μ raxy magnesium acetate rcayevd shake and yellow crystalline ppt forms Na+ absent. |
| ii) For the next part carry out the test with Na2HpO4 white ppt forms Mg++ present. | ii) Add a little sodium cobaltinitrite solution and a few drops of dil.acetic acid no yellow ppt forms. |
| iii) K+ absent. |
Reaction Involved:
White crystalline ppt.cf magnesium ammonium phosphate or presence of ammonium chloride an ammonia solution to prevent precipitation of MgSO4 + Na2HpO4 ———–> MgHpO4 + Na2SO4
RESULT:
Hence, the acid radicals were found to be Cl– and CO3—and basic radicals were found to be Fe++ and mg++.
CONCLUSION:
Hence, the complete analysis of given salt was done in wet ways and dry ways in the laboratory.
PRECAUTION:
i) Chemicals should be handled carefully.
ii) Group separation should be done carefully.
iii) Reagents should be taken in the proper amount.
iv) Heating of solution should be done carefully.
