APPARATUS REQUIRED:
a) Beaker
b) calorimeter
c) Thermometer
CHEMICALS REQUIRED:
a) KNO3
b) Water
THEORY:
Heat of solution (enthalpy) is defined as the quantity of heat absorbed or evolved when 1 mole of Substance is dissolved in excess volume of solvent and heat of solution does not change on further dilution. In order to determine the enthalpy of the calorimeter i.e. the heat capacity of the calorimeter is to be determined as it mentioned previously. If the system decreases its temperature during dissolution, the process would be endothermic. But if the system increases its temperature during dissolution, the process would be exothermic. In endothermic process heat change by water and calorimeter would have -ve sign and in exothermic reaction process, It would be +ve sign
PROCEDURE:
At first 200 gm of water was taken in a polystyrene cup and initial temperature was recorded with the help of a thermometer. Then the mole ratio of solvent to solute i.e 200g was taken. Then about 55g of KNO3 was added to a polystyrene cup containing 200 of water. The solution was stirred continuously with the help of glass rod until complete dilution.The final temperature was recorded.
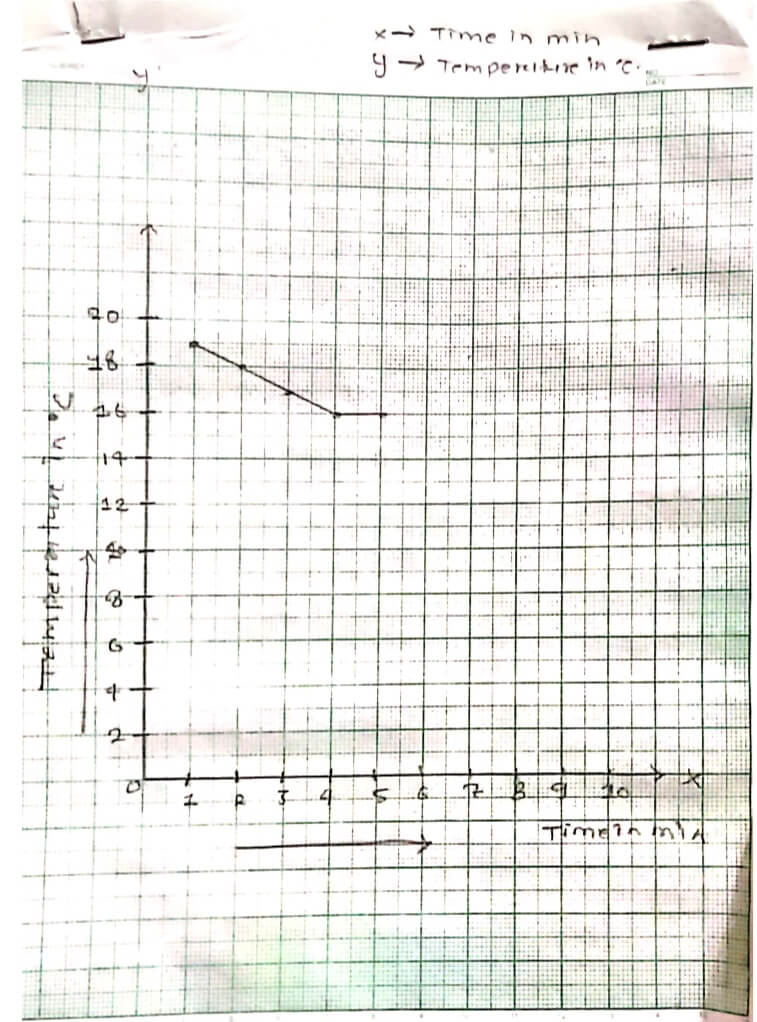
OBSERVATION:
| S.N | Observation of KNO3 | |
| Time in minute | Temperature in °C | |
| 1. | 1 minute | 19°C |
| 2. | 2 minute | 18°C |
| 3. | 3 minute | 17°C |
| 4. | 4 minute | 16°C |
| 5. | 5 minute | 16°C |
observation:
Mass of water (M3) = 200 g
Initial temperature (t1c) = 19°C
Amount of solute KNO3 (w2) = 5.5 g
Final temperature (t2c)=16°C
Water equivalent (w) = 62.7 J/g
Now, By using formulas of heat change.
Total heat change = M3-5(t₂-t1)°C+ w (t3-t1)°C x molecut wt / w1
= 200 x 41 (16-19) +62.7(16-19) x104 / 5.5
= -26961 / 5.5
= -50490.6
= -50.4 kj
i.e △H = -50.4 kj
RESULT:
Hence, Heat (enthalpy) of the solution of KNO3 was found to be △H=-50.4 kj
CONCLUSION:
Hence, Heat (enthalpy) of solution can be calculated by calorimetrically.
PRECAUTIONS:
1) Time should be measured accurately.
2) Apparatus should be handled carefully.
