APPARATUS REQUIRED:
a) Beaker
b) Calorimeter
c) Thermometer
CHEMICALS REQUIRED:
a) Dil HCL
b) Dil NaOH
c) Water
THEORY:
A thermodynamic equation to neutralize strong acid and strong base is given as follows.
HCl(aq) + NaOH (aq) ———-> NaCl(aq) +H2O △H = -Q.
Tonic thermodynamic equation for neutrally or neutralization reaction is.
H+(aq) + OH–(aq) ———-> H2O(l), △H = Q.
where -Q is heat of neutralization per equivalent Acid base reaction.
Heat evolved in neutralization raises the temperature of aqueous solution and calorimeter. Therefore, heat of neutralization is determined by finding out the quality θ heat gained aqueous solution and calorimeter containing 1g. equivalent of acid and 1g equivalent of
Then,
Heat lost by water =heat gained by cold water+calorimeter.
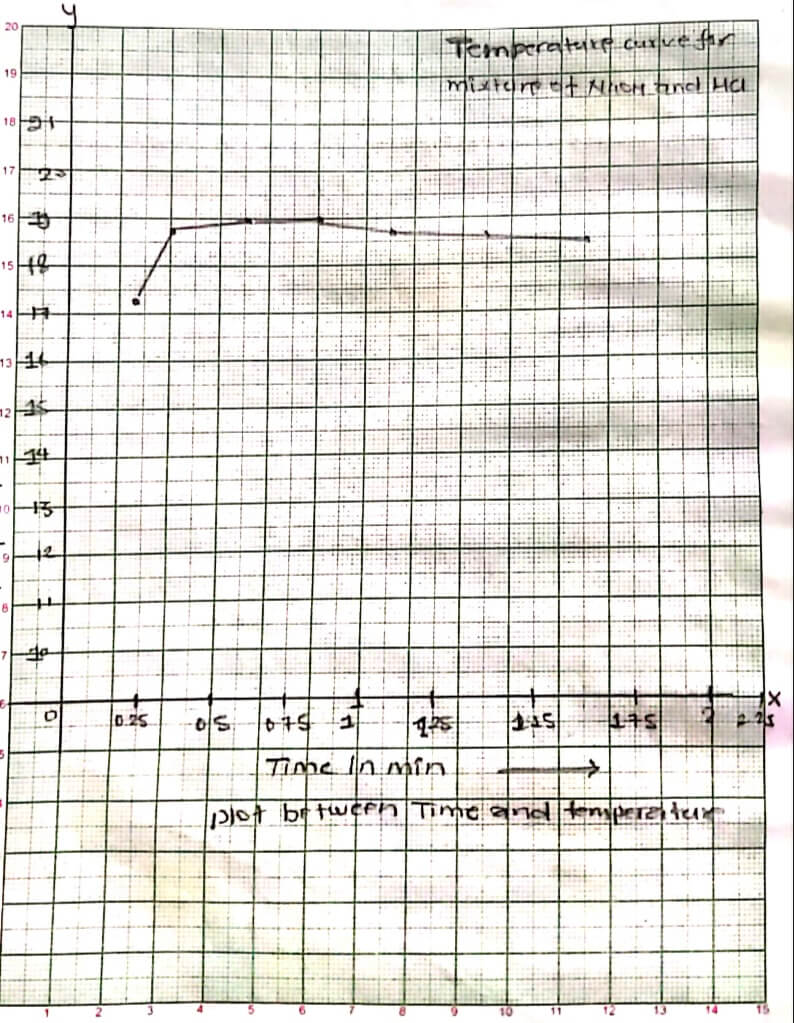
PROCEDURE:
At first 50 ml of HCl and 50 ml of NaOH was taken in a beaker Separately. The initial temperature of HCl and NaOH was calculated.Then they were mixed in a single calorimeter and temperature was noted. The temperature was noted for about 5 times for 5 min.until a constant temperature was obtained. For specific heat capacity of calorimeter, cold water and hot water temperature were noted and formulas were applied with the help of this of neutralization was calculated.
OBSERVATION:
For calorimeter:
specific heat of water 5=4.18
mass of hot water (m1) = 50gm
mass of cold water (m2) = 50 gm
Temperature of hot water t₁ = 64°C.
Temperature of cold water t2 = 18°C
After mixing
Final temperature t3 = 38°C
decrease in temperature of hot water =t₁ – t3
=64°C – 38°C
= 26°C
Increase in temperature of cold water = t3 – t2
= 38°C-18°C
= 20°C
= △t2
Then,
Heat lost by water = Heat gained by cold water + calorimeter
m1S (△t)1 = m2S (△t)2 + w(△t2)
W = m1S(△t₁) – m2S(△t2) / (△t)2
= 50 x 4.18 x 20 – 50 x 4.18 x 18 / 20
= 5434 – 4180 / 20
= 1254 / 20
= 62.7
Therefore, the water equivalent was found to be 62.7 J/mol.
OBSERVATION TABLE:
| O.T of HCL | O.T of NaOH | O.T OF Mixture | |||
| Time in minute | Temperature in °C | Time in minute | Temperature in °C | Time in minute | Temperature in °C |
| 1 minute | 19°C | 1 minute | 19°C | 1 minute | 24°C |
| 2 minute | 19°C | 2 minute | 19°C | 2 minute | 24°C |
| 3 minute | 19°C | 3 minute | 19°C | 3 minute | 24°C |
| 4 minute | 19°C | 4 minute | 19°C | 4 minute | 24°C |
| 5 minute | 19°C | 5 minute | 19°C | 5 minute | 24°C |
Now,
For neutralization:
Temperature of NaOH solution t₁ = 19°C
Temperature of HCl solution t₁ = 10°c
Final temperature of mixture = t2.
volume of acid V₁ = 50ml
Volume of bast V2 = 50ml
Heat evolved in reaction = [V₁ + V₂] 4.13 [T₁-T2] +w [T₁-T2]
= 2403.5
For 1000m 1 of 1m HCl = 1000/V₁ XA
=1000/50 x (-2403.5)
= 48.07kg
RESULT:
Hence the heat of neutralization of acid and base was found to be 48.07kg.
CONCLUSION:
Hence, the heat of neutralization of acid and base can be calculated from the above experiment.
PRECAUTION:
1) Time should be measured accurately
2) Apparatus should be handled carefully.
