APPARATUS
Laurent’s half – Shade polarimeter , a sodium lamp sugar, a balance weight box, a graduated cylinder, two beakers, filter paper, a funnel, an eyepiece, a pipette and a glass rod.
THEORY
Light in which vibrations are symmetrically distributed about the direction of propagation of an ordinary Light. When light acquires the property of one sidedness due to reflections, refraction and transmission through a crystal it is said to be polarized and this phenomenon of acquiring one- sidedness with respect to intensity is called polarization.
Laurent’s half-shade polarimeter : It is an instrument used for finding the optical rotation of sugar; it is called a Saccharimeter. If the specific rotation of sugar is Known the concentration of the Sugar solution can be determined.
Constructions: The optical parts of a polarimeter are shown in fig. in which S is a source of monochromatic light usually a sodium lamp. The light from the source after passing through a narrow slit is rendered into a parallel beam by the lens ‘L’ ; the light is made plane polarized.
By nicol prism p after passing through the half-shade device H (described later) a glass tube T containing the solution is made to fall on the analyzing nicol A. The light is viewed through a galilean telescope E. The analyzing nicol A. can be rotated about the exis of the tube and it’s rotation can be Measured on the graduated circular disc ‘c’ divided in degree with the help of a vernier.
If θ be the optical rotation produced by ‘I’ decimeters of a solution and C the concentration in gram per C-C then specific rotation, S at a given temperature ‘t’ and corresponding to wavelength λ is given by
[S]tλ = θ/λc= Rotation in degree / length in decimeters x concentration in gm/cc
OBSERVATIONS
Weight of Sugar m = 5 gram
Volume of solution required = M/20 x100%
= 100 x 5 /20
= 25cc
Vernier Constant:
10 division of vernier scale coincides with 9 division of mainscaled
1 division of vernier scale coincides with 9/10 division of mainscaled
Vernier Constant = 1-1 division of Vernier scale
= (1- 9/10)x10
= 1/10 x 1°
= 0.1°
Length of tube = 21.5cm
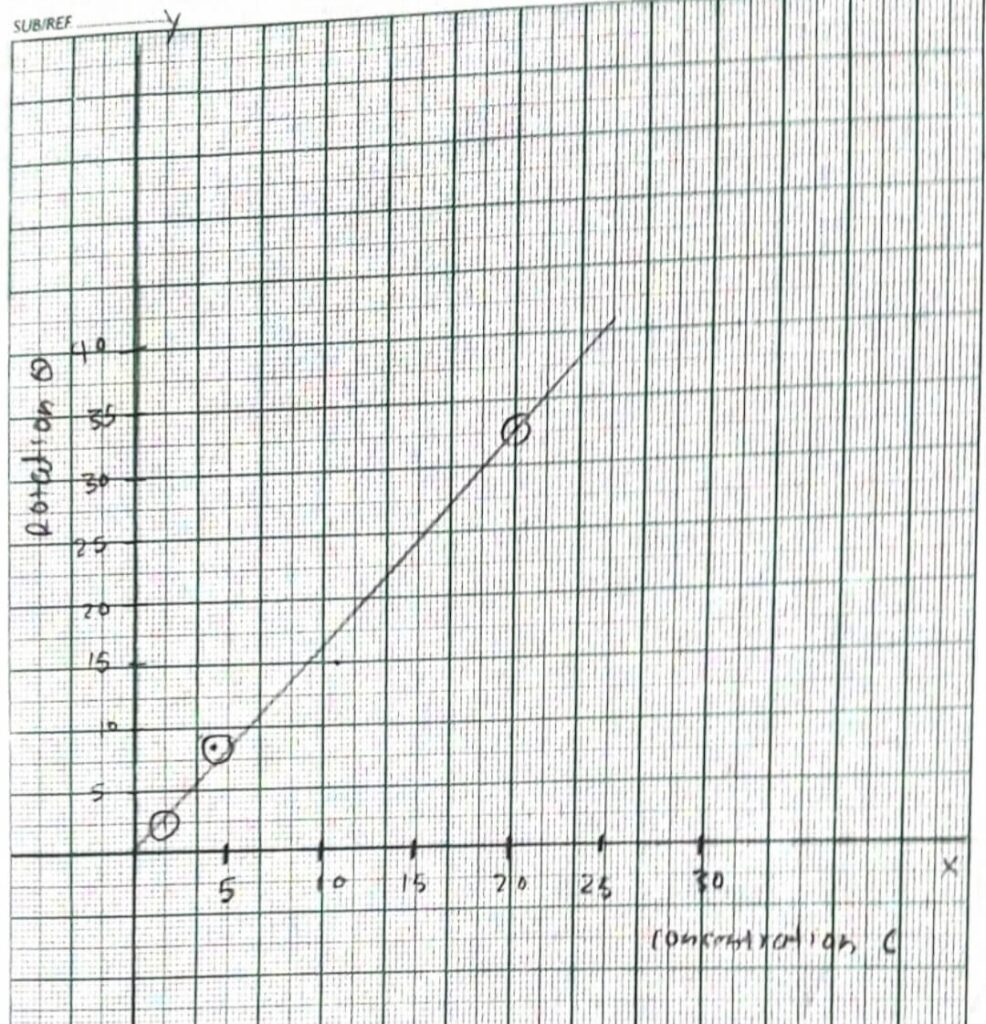
OBSERVATION TABLE
Volume of Solution required 25cc
| S.N. | Strength of solution in gram per 100cc | Strength of solution in gram per cc | Scale reading through solution 1st | Scale reading through solution 2nd | Rotation 1st | Rotation 2nd | θ/c |
| 1 | zero | 0 | 161.5 | 341.2 | 0 | 0 | 0 |
| 2 | 20 | o.2 | 189.9 | 309.7 | 28.4 | 31.5 | 29.35 |
| 3 | 10 | 0.1 | 176 | 355.4 | 19.5 | 14.2 | 14.35 |
| 4 | 5 | 0.05 | 168.5 | 348.5 | 7 | 7.5 | 7.15 |
| 5 | 2.5 | 0.025 | 165.1 | 344.9 | 3.6 | 3.7 | 3.65 |
therefore, Stλ1 = 10xθ/V.C = 10×0/21.5 = 0
Stλ2 = 10xθ/V.C = 10×149.75/21.5 = 69.65°
Stλ3 = 10xθ/V.C = 10×143.5/21.5 = 66.74°
Stλ4 = 10×143/21.5 = 66.5°
Stλ5 = 10×146/21.5 = 67.90°
Stλ mean = 69.65° +66.74° +66.5° +67.90° / 4
= 67.69
Standard Value = 66.7
% Error = |(standard – observed) value /Standard value| x 100
= (66.7-67.69) / 66.7 x 100
= 1.48%
PRECAUTION
i) The tube and glass window should be clean.
ii) There should be no air or air bubble in the glass tube when the tube is filled with the solution.
iii) The cap should be screwed in such a manner that there is no leakage · These should not be made very tight so as to strain the glass windows.
Explore More Physics Practicals | B.Sc. 2nd Year | T.U.
- Zener Diode Characteristics & Breakdown Voltage | Experiment Guide
- Determine Specific Rotation of Sugar Solution Using a Polarimeter
- Find Impedance of LCR Series Circuit & Calculate Quality Factor
- Determine Operating Voltage of G.M. Counter | Schematic & Guide
- Verify NAND Gate as a Universal Gate Using IC Chips
- Study NPN Transistor Characteristics in Common Base Mode
- Find Wavelength of Light Using a Diffraction Grating
- Find Wavelength of Sodium Light Using Newton’s Rings
- Determine Viscosity Coefficient of Water | Poiseuille’s Method
- To Determine Surface Tension Using Capillary Tube Method
- Determine Young’s Modulus by Bending Beam Method | PDF
- Determine Moment of Inertia & Angular Acceleration | Flywheel
- Determine Acceleration Due to Gravity & Radius of Gyration | Bar Pendulum
- Determine Modulus of Rigidity Using Maxwell’s Needle | PDF
- Study Charging & Discharging of Capacitor | Find Capacitance
