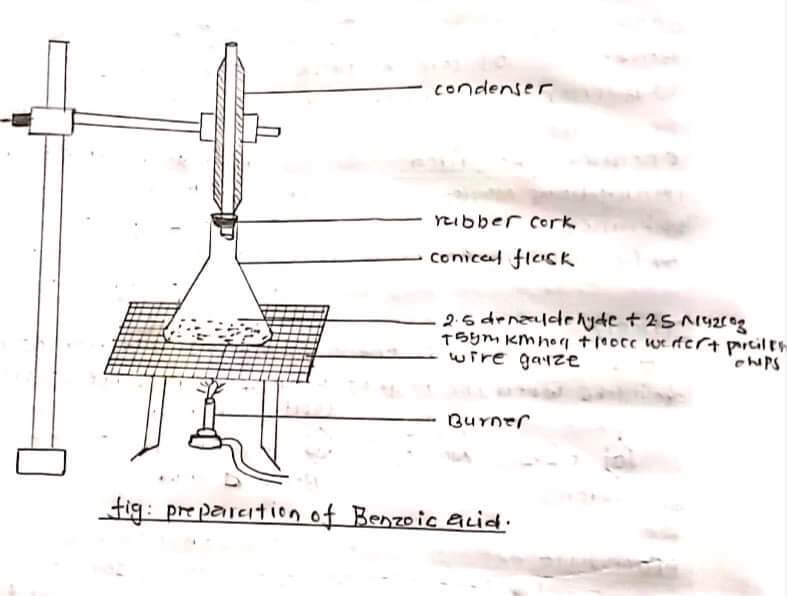
APPARATUS REQUIRED:
a) Beaker
b) Conical flask
c) condenser
d) Burner and funnel
e) Glass rod
CHEMICALS REQUIRED:
a) Anhydrous Na2CO3
b) kmnO4
c) Benzaldehyde
THEORY:
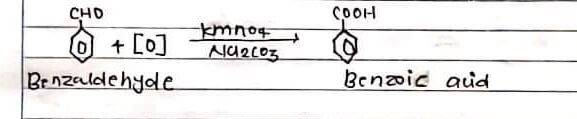
A benzoic acid is prepared by the addition of benzaldehyde with the help of kmn04 in presence of alkali.
PROCEDURE:
5 ml benzaldehyde 5 gm Na2CO3 10g of 100 ml of water and porcelain chips was taken in a conical flask, the mixture was refluxed using a water condenser i.e about 45-1 1/2 hours. The mixture was cooled and filtered with the help of filter paper. In this filtrate solution conc. H2SO4 was added till complete ppt was obtained and filtered. After filtration washed the ppt with 200m of water and a small amount of charcoal was added. Then boiled this solution and filtered the solution through glass rod and funnel with benzoic acid would crystallize out as colorless Crystal from on cooling the filtration and washed it with a cold water and semi-dried between the folds of filter paper.
RESULT:
Hence, from above experiment white shining crystals obtained having melting point 121°c.
CONCLUSION:
Hence, the white shining crystals can be obtained by oxidation of benzaldehyde with the help of kmnO4 in the presence of alkali.
PRECAUTIONS:
1) Apparatus should be handled carefully.
2) Cooling process should be done carefully.
3) Conc. HaSO4 should be handled carefully.
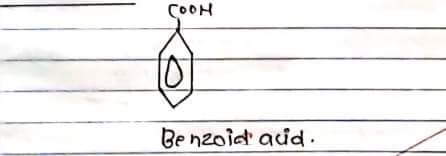
STRUCTURE: