APPARATUS REQUIRED
i) Test tube
ii) Test tube holder
iii) Test tube stand
iv) Tong
CHEMICAL REQUIRED
i) Silver Nitrate.
ii) Crystals of KMnO4
iii) Chloroform
iv) Hydrochloric acid.
THEORY
Qualitative analysis of an inorganic salt is the detection of acid and basic radicals of a salt by suitable method.
A salt is simply the compound which is formed by Complete or partial neutralization of acid by a base. Salts are strong electrolytes. When dissolved into positively and negatively charged ions.
Acid radicals are simply the electronegative radical obtained from acid in the salt. Eg:- Cl–, NO3—, CO3— but the exception is OH– ion which is only electronegative radical but not acidic radical.
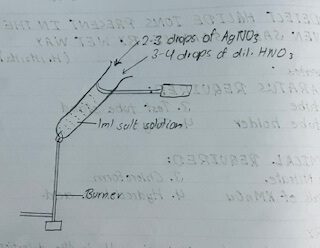
Wet ways:
The test performed on the form of solution by dissolving a salt on a suitable solvent are called wet ways.
During wet tests the radicals are selected from the solution generally by observing the characteristics color of the precipitate or solution or gas formed after the conic interaction with the Suitable reagent.
SAMPLE NO: H₁
Physical properties:
State ————-> crystalline solid
Colour ————> White
Odour ————–> Characteristic
Solubility————> Soluble in water
PROCEDURE
The original solution (0.5) was made by dissolving the given sample in water and difference test were performed as:-
OBSERVATION TABLE
| Experiment | Observation | Inference |
| Few ml of prepared solution was taken in test tube and few drops of AgNO3 a solution was added. | Curdy white ppt was formed. | presence of Cl– ion. |
REACTION INVOLVED
Cl + AgNO3 —————> Ag Cl ↓+ NO3–
(white ppt)
RESULT
Hence, In the given inorganic salt sample (H) was chloride (Cl–).
SAMPLE NO: H₂
Physical properties:
State ————> crystalline solid
Color ———–> White
Odor ————> Characteristic
Solubility ———> soluble in water
OBSERVATION TABLE
| Experiment | Observation | Inference |
| Few ml of prepared solution was taken in test tube and Few drops of AgNO3 solution was added. | light yellow ppt wasformed. | May be presence of Br– ion. |
CONFIRMATIVE TEST FOR SAMPLE NO: H₂
| Experiment | Observation | Inference |
| Few ml of 0.5 was taken in the test tube, a few drops of conc. HCI and a small crystal of KMnO4 was added and a few ml of chloroform was added and was shaken well. | The bottom layer of chloroform wasbrown coloured. | Presence of Bromide(Br–). |
REACTION INVOLVED
Br + AgNO3 ————> Ag Br + NO3–
(pale yellow)
REACTION OF CONFIRMATIVE TEST:
KMnO4 + Conc. HCl ————> KCl + Mn Cl₂ + H₂O [l]
2Br + 2[Cl] ——————–> Br₂ + 2Cl–
Br2 + CHCl3 ——————> Brown colour chloroform lager was seen at the bottom.
RESULT
In the given SAMPLE NO: H₂ Bromine (Br–) was found.
SAMPLE NO: H3
Physical properties:
State ————> crystalline solid
colour ————> white
Odour ———–> characteristics
Solubility ———-> soluble in water
OBSERVATION TABLE
| Experiment | Observation | Inference |
| Few ml of prepared solution was taken in the test tube and Few drops of AgNO3. | Yellow ppt was formed. | May be presenceof I– ion. |
CONFIRMATIVE TEST FOR SAMPLE NO: H3
| Experiment | Observation | Inference |
| Few ml of O.S. was taken in test tube, Few drops of cone HCL and small crystal of KMnO4 was added and few ml of chloroform was added and was shaken well. | The bottom layer of chloroform was violet coloured. | presence of Iodide(I–). |
REACTION INVOLVED
I+ AgNO3 ———–> AgI + NO3–
(yellow ppt)
CONFIRMATIVE REACTION TEST OF H3
KMnO4 + conc. HCl ————> KCl + MnCl2 + H2O + [CL]
2I– + 2[CL] ———-> I2 + 2CL–
I₂ + CHCl₂ ————> Violet color chloroform lay was seen at one bottom
RESULT
Hence, In the given inorganic salt SAMPLE NO: H3 was Iodide (I).
CONCLUSION
In this way, the liquid concentration present in a given sample can be detected by wet ways.
PRECAUTIONS
1. Apparatus should be heated and cleaned.
2. Reagents used should be an accurate amount.
3. Conc. HCL should be handled carefully.
