Apparatus Required
i) Test tube
ii) Test tube holder
Chemical reactions
i) KMnO Solution
ii) Mg ribbon
iii) SnCl2
iv) Copper tuning
v) H2SO4
vi) Kn
vii) K₂CrO2 Solution.
viii) HgCl2
ix) Conc.HNO3
x) HCl
THEORY
Redox reactions are those reactions that involve oxidation half reaction and reduction half-reaction occurring simultaneously in the same reaction. ‘So oxidation and reduction undergues’
Simultaneously
Redox reaction can be defined in term of
1. Tone concept.
2. Oxidation number concept.
Oxidation:
It is the simple case of an election or increase in positive charge or decrease charge by an atom or ion car simply increasing ion oxidation. It is also called de- electronation.
Reduction:
It is simply gain of elections or decrease in position change.or increase in negative charge by on atom or ion or simply decrease in oxidation number. It is also called electronation.
Oxidizing agent (oxidant):
“It is the species in a chemical reaction which oxidizes others and undergoes it self reduction”.
Reducing agent (Reduction):
“It is the species in a chemical reaction which reduces others and undergoes it self oxidation”.

PROCEDURE
The following test are performed as:
OBSERVATION TABLE
| S.N. | Experiment | Observation | Inference |
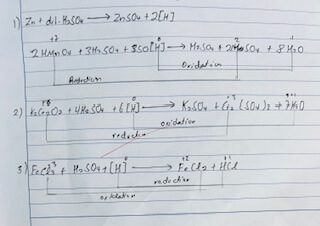
| 1. | A piece of granulated Zn was dropped into a test tube containing acidified KmNO4 and shaked. | The park color of KMnO4 was discharge to colorless. | Due to the reduction of KMnO4 into MnSO4. |
| 2. | A piece of granulated Zn was dropped in the test tube of acidified k2Cr₂O7 solution and shaked. | Orange color of k2Cr₂O7 was changed into light green. | Due to the reduction of k2C₂O7 to Cr2(SO4)3. |
| 3. | A piece of granulated Zn was dropped to the solution of acidified FeCl3 and shaked. | Yellow color of FeCl3 changed into light green. | Due to reduction of FeCl3 to FeCl2. |
| 4. | Magnesium ribbon wasburnt in air. | White ash was produced. | Due to the oxidation of Mg to MgO. |
| 5. | Copper turns were treated with Conc. HNO3 taken in the test tube. | Brown fumes of NO₂ gas evolved. | Due to oxidation number of Cu into Cu(NO3)2 and NO2 reduction of HNO3 to NO₂. |
| 6. | Granulated zinc piece was dropped into the gas for containing dil. H₂SO4. | Burning matchstick was introduced into the testube involved from the reaction which produces pop sound. | Due to the oxidation of Zn to ZnSO4 andliberation of H2 which is reduced. |
| 7. | Potassium iodine solution (HI) was treated with CuSO4 solution taken in test tube. | Brown ppt was formed. | Due to reduction of K2HnO4 to KHnSO4. |
| 8. | Mercuric chloride (HgCl2) solution was treated with SnCl2 little by little till excess. | Grey ppt. First was formed then was changed to black. | Due to reduction of HgCl2 into Hg2Cl2 and finally to free Hg. |
Reaction INVOLVES

RESULT
Hence, some redox reactions were studied.
CONCLUSION
In this way redox reactions can be studied.
PRECAUTIONS
i) Apparatus should be handled carefully.
ii) Limited amount of chemicals should be used.
iii) Chemical should be handled carefully
