APPARATUS REQUIRED:
i) Round bottom flask
ii) Gas Jar
iii) Test tube stand
iv) Tripod stand
v) Delivery tube
vi) Cork
vii) Test tube
viii) Wire gauge.
CHEMICALS REQUIRED:
i)Ammonium chloride (NH4Cl)
ii) Calcium hydroxide or slaked lime Ca(OH)2
iii) Conc. HCl
iv) CuSO4 solution
v) FeCl3 solution
vi) FeSO4 solution
vii) Mercurous nitrate solution
viii) Phenolphthalein solution
THEORY:
Ammonia gas is prepared in laboratory by heating the mixture of ammonium chloride (NH4Cl) and calcium hydroxide Cu(OH)2 in the ratio 1:2 by weight as:
triangle
2NH4 Cl+ Cu(OH)2 —————–> CuCl2 + 2NH3 ↑ + 2H₂O
Ionic reaction
NH4+ (aq) + OH–(aq) ————>NH3 ↑ + H₂O
The gas formed is collected underwater as it is in the gas jar by downward displacement of air, as the gas is lighter than air.
The gas cannot be collected underwater as it is highly soluble in water. If dry ammonia gas (NH3) is to be prepared than ammonia gas should be dried by passing through lime towers but anhydrous CuCl2 as well as Conc. H₂SO4 cannot be used as a drying agent, it is because they react with ammonia.
NH3+ Conc. H₂SO4 ——————> (NH4)₂SO4
Anhydrous CaCl2 + NH3 ————-> CaCl₂. 8NH3
[octa anime calcium chloride]
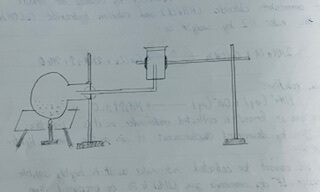
PROCEDURE:
The mixture of ammonium chloride (NH4Cl) and calcium hydroxide Ca(OH)₂ in the ratio 1:3 by weight in the form of paste was taken in a round bottom flask. Then the flask was fitted with a delivery tube and set in a slight slanting position supported by a stand with clamp as shown in figure. The whole mixture was then heated, ammonia (NH3) gas produced was passed through the delivery tube and finally collected in the gas jar by downward displacement of air. Thus, collected NH3 gas was used to study its properties.
OBSERVATION TABLE:
| S.N. | Experiment | Observation | Inference |
| 1. | Color of the was noted. | No color was observed. | Ammonia gas is colorless. |
| 2. | Odor of gas was tested. | Ringent smell was noted. | The Ammonia gas is pungent in smell. |
| 3. | Moist red and blue litmus paper were introduced to the gas. | Red litmus paper, turnedto blue but most bluewas observed to notchange in color. | Ammonia gas is basic in nature. |
| 4. | Burning match stickwas introduced to the gas. | The match was extinguished and gas was observed to not burn. | Ammonia gas is neither combustible nor a supporter of burning. |
| 5. | A gas jar filled with NH3 gas was inverted over water and was shaken up and down. | Water level was observed rising inside the gas jar. | Ammonia gas is highly soluble in water. |
| 6. | NH3 gas was passed in a test tube containing CuSO4 solution for a short time. | Bluish white ppt was obtained. | Cupric hydroxide Cu(OH)2 is formed. |
| 7. | Few drops of conc. HCl was taken in a test tube and NH3 gas was passed into it. | Dense white fumes were observed. | Ammonium chloride is formed. |
| 8. | The NH3 gas was passed in a test to be containing CuSO4 solution for long time. | Brown ppt. was observed. | Ferric hydroxide Fe (OH)3 is formed. |
| 9. | Ammonia gas was passed through the FeSO solution. | Dirty green ppt.was obtained. | Ferrous hydroxide Fe (OH)2 is formed. |
| 10. | Ammonia gas was passed over wet-filter paper with mercuric nitrate solution. | The wet filter paper was observed turning into black. | Monobasic ammonium mercuric nitrate and finely divided mercury is formed. |
| 11. | Ammonia gas was passed into Nessler’s reagent test tube. | Reddish brown ppt was observed. | Millon’s base is formed. |
| 12. | The gas was passed into a few drops of phenolphthalein solution in a test tube. | Colorless phenolphthalein was turned into pink. | Ammonia gas is basic in nature. |
| 13. |
REACTION INVOLVED:
NH3 + H2O ————> NH4OH —right left harpoons—— NH4+ + OH–
Ammonium hydroxide
NH3 + Conc. HCL —————-> NH4Cl
Ammonium chloride
(dense with fumes)
For short time
CuSO4 + 2NH4OH ———————–> Cu (OH)2 ↓+ (NH4)2SO4
Cupric hydroxide
(Bluish white ppt)
long time
Cu(OH)2 + (NH4)2SO4 + NH4OH ——————> [Cu(NH3)4] SO4 +H₂O
[Tetraamine copper (11) sulpbate ]
(Deep blue colour)
FeCl3 + 3NH4OH —————–> Fe (OH)3 ↓ + (NH4)2 SO4
Ferrous hydroxide
(Dirty green ppt)
Hg2(NO3)2 +2NH3 ————–> [Hg + Hg (NH₂) NO3 ] + NH4 NO3
[ Mercuric Amino Nitrate ]
(Black ppt)
NH3 + 2KHgI4 + 3KOH ———–> NH2Hg OHgI + 7KI+2H2O
[Millon’s base]
(brown ppt)
RESULT:
Hence, Ammonia gas was prepared in the laboratory and it’s properties were studied..
CONCLUSION:
In this way, Ammonia gas can be prepared in the laboratory by heating the mixture of NH4CL and Ca(OH)2 and its properties can be studied.
PRECAUTIONS:
1) The apparatus should be neat and clean.
2) Apparatus must be handled carefully.
3) The apparatus should be airlight.
4) Heat should be given gently.
5) R.B Flask should be clamped at an incline
