APPARATUS REQUIRED
i) Beater
ii) Porcelain Basin
iii) Glass rod
iv) wire gauze
v) Test tube
vi) Filter paper
vii) Tripod stand
CHEMICAL REQUIRED
i) Barium Chloride [BaCl2 solution]
ii) Dilute sulphuric acid (dil H₂SO4)
iii) AgNO3 solution
THEORY
Precipitation reaction is a double displacement reaction in which the constituents of reactant are interchanged and hence water insoluble compound is formed from a water soluble compound. When BaCl2 solution is treated with dil. H₂SO4 solution in soluble BaSO4 is obtained as white precipitate.
BaCl2 + dil. H₂ SO4 ———-> Ba. SO4 ↓ + 2HCl
[White ppt]
i) The insoluble substance produced during the process is called precipitate. Here, BaSO4 precipitates.
ii) The constituent which is responsible for precipitation is called precipitant Here, H₂SO4 is precipitant to occur ionic product [K,p] should be a greater solubility product.
iii) For the precipitation to occurs ionic product, [K,P] should be greater than solubility product [k, P]
iv) During the precipitation reaction the clear solution left above precipitates in called supernatural liquid.
v)The impurities mainly present in this process are chloride (Cl–) and sulphate [SO4–]. The purity of precipitate can be checked by treating the filtrate obtained after washing with AgNO3 solidon and BaCl2 solution respectively.
Cl– + AgNO3 ———> AgCl ↓ + NO3–
[Curdy white ppt]
SO4— + BaCl2 ————-> BaSO4 ↓ + 2Cl–
[White ppt ]
PROCEDURE
About one test tube full of BaCl2 solution was taken in beaker and dil. H₂SO4 was added to it. Half test tube of dilute H₂SO4 was added to the BaCl2 solution and was stirred well. Heat the mixture gently such that BaSO4 settles down completely obtaining a clean solution above. This solution is called supernatant liquid. The completeness of the reaction was checked by taking little supernatant liquid in the test tube and adding little H₂SO4. If ppt was obtained then again little dil. H₂SO4 was added to the mixture. The process continued till no ppt. was seen. Then the mixture was filled and it was washed several times with water. The impurities present were tested by using AgNO3 solution and BaCl2 solution after each impurity was then taken out and dried in the oven or flame. It was submitted for inspection.
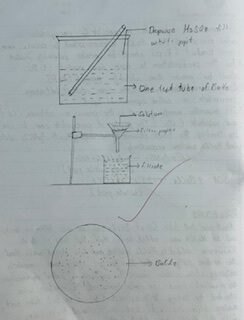
OBSERVATION TABLE
1. Purity test of Cl
| S.N. | Experiment | Observation | Inference |
| 1. | Few ml of filtrate was taken in test tube and few drops of AgNO3 solution was added. | Curdy white ppt was obtained. | Due to presence of Cl– ion in precipitate. |
| 2. | After several washing, few ml of filtrate was taken in test tube and few drops of AgNO3 added. | white ppt was not obtained. | Due to absence of Cl– ion in precipitate, |
TEST REACTION
Cl– + AgNO3 ————> AgCL ↓+ Cl–
[Curdy white ppt]
RESULT
Hence, pure and dry BaSO4 ppt was obtained and was submitted for inspection.
CONCLUSION
In this way, the precipitate of BaSO4 in pure and dry state can be obtained by precipitation followed by filtration.
PRECAUTIONS
i) Apparatus should be handled carefully.
ii) Acid should be added little by little with constant stirring.
iii) Fast deposition can be done by slight heating.
iv) Impurity tests should be done carefully.
v) Complete ppt. should be checked by carefully.
