APPARATUS REQUIRED
i) Porcelain basin
ii) Beaker
iii) Glass rod
iv) Test tube
v) Funnel
vi) Wire gauze
vii) Burner
viii) Filter paper
ix) Spatula
CHEMICALS REQUIRED
Impure bazaar copper sulphate
THEORY
The pure blue vitriol crystals (CuSO4.5H₂O) are removed from the saturated solution of bazaar copper sulphate by crystallization.
Solution:
A solution is a homogenous mixture of two or more substances. A solution has parts which are solute and solvent.
Saturated solution:
The solution which cannot dissolve more solute at the given temperature is called saturated solution.
Unsaturated solution:
The solution which can dissolve more solute at a given temperature is called an unsaturated solution.
Supersaturated solution:
The saturated solution which is prepared by dissolving excess solute at high temperature which on cooling gives cut the precipitate is called supersaturated solution.
Salute:
The solute is the substance that dissolves in solvent. eg. sugar, alcohol, etc.
Solvent:
The majority of the solution which can dissolve solute is called solvent, eg. excess water, alcohol etc.
Crystallization:
The process of formation of crystals from the saturated solution is called crystallization from its saturated solution followed by sudden costing of excess dissolved solute of its crystalling point is called crystallization.
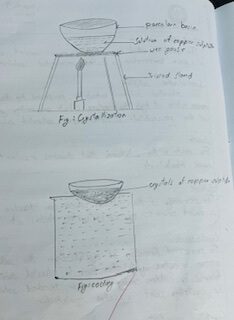
Crystallization point:
The temperature at which the fine crystals just start to form during the crystallization process is called Crystallization point.
Crystals:
The pure solid has a definite geometric shape and size of the particles in the regular pattern called crystals.
Mother liquor:
The clear solution left after the formation of crystals, during crystallization it is called mother liquor. Water of crystallization CuSO4. 5H₂O
The no. of water molecules associated to the one molecule of crystals is called water of crystallization,eg. Na₂CO3. 10H₂O.
CuSO4—right left harpoons——–>CuSO4. 5H₂0
(Solution) (Blue vitriol)
PROCEDURE
The sample of bazaar copper sulphate (CuSO4) was taken in a beaker and half a test tube of water was added in it. The mixture in the beaker was stirred using a glass rod to prepare a saturated solution. The solution was filtered through filter paper kept on a funnel in a porcelain basin to separate insoluble impurities. The mixture in the basin was placed over a tripod stand with a wire gauge and was heated up to the crystallization point. In order to test whether the solution has reached the crystallization point or not, droplets of solution were touched on the wall of the test tube filled with four fifths of water. The droplets were taken with the help of glass rod from the boiling solution When mini crystals were observed on the wall of the test tube, the crystallization point of solution was identified. The burner was turned off and the porcelain basin was placed over the beaker which was completely filled with water. After some time, crystals of blue vitriol were observed and were scraped from the basin using spatula on a filter paper.
OBSERVATION
| S.N. | Experiments | Observations | Interfere |
| 1. | Few drops of boiling saturated solution of bazaar copper sulptate were touched on the walls of the test tube Controlling fourth- fifth of water. | Mini crystals not observed on the walls of test tube. | Crystallization point is not reached. |
| 2. | After few minutes, again the solution was touched to the test tube containing water. | Tiny particles were appeared on the outer walls of the test tube. | Crystallization point is reached. |
RESULT
The crystals of blue vitriol were obtained from the bazaar copper sulphate.
CONCLUSION
The crystals of blue vitriol are obtained from the bazaar copper sulphate through the process of crystallization.
PRECAUTIONS
i) The apparatus should be handled carefully.
ii) The filtrate should not contain any impurities.
iii) The crystallization point should be checked from time to time by heating the solution must be saturated.
iv) Without disturbing the porcelain basin which contains solution should be placed over the beaker completely filled with water.
