APPARATUS REQUIRED
i) Porcelain basin
ii) Funnel
iii) Filter Paper
iv) Wire gauze
v) Tripod stand
vi) Burner
CHEMICAL REQUIRED
i) Sand and Camphor
THEORY:
The mixture of volatile and non-volatile components can be separated by sublimation process.
Mixture
A Mixture is a combination of two or more substances without changing their identities.
Volatile substance
These substances which can directly convert into vapour without any intermediate phase at ordinary temperature due to their low boiling point are called volatile substances eg: camphor, Iodine, petrol etc.
Volatile component is separated from the mixture of volatile and non volatile solids by sublimation process.
Volatile substance:
The substance which evaporates at normal temperature is known as volatile substance. For eg, camphor, ammonium chloride, iodine, alcohol etc.
Non-volatile substance:
The substance which does not evaporate at normal temperature is known as non volatile substance Example: sand e.t.c.
Sublimation:
The process in which a solid is directly converted into its vapor on healing and the vapor back into solid on cooling without changing an intermediate liquid state is called sublimation.
Sublimate:
The substance which is obtained after the sublimation process is called sublimate eg. Camphor
PROCEDURE :
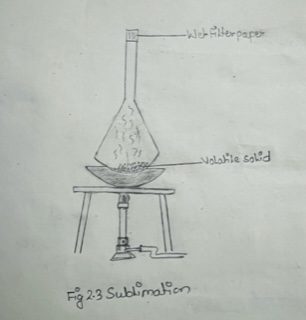
The given mixture of sand and camphor was taken in a clean and dry porcelain basin. A glass funnel was wrapped by method filter paper in the outer surface and blocked the hole of the stem from the inner side by the dry cotton. The sample was covered by funnel and kept over the triped stand with wire gauze. The burner was introduced to heat the porcelain basin fill 2-3 min. The funnel was moved from the porcelain basin and the pure camphor ceased, healing was discontinued and the adjustment was cooled by air cooling. The funnel was removed from the porcelain basin and the pure camphor was scraped by spatula into a water glass and submitted for inspection.
The porcelain basin was cooled and sand was recovered.
OBSERVATION:
| S.N. | Experiments | Observation | Inference |
| 1. | The separate sand of Smell of porcelain basin was smelled. | smell of camphor was found | Sand is not freefrom the camphor. |
| 2. | The sand was heatedstrongly continuous stirling with glass rod and smelled. | No smell of camphor was found | Sand is pure |
RESULT :
The camphor were separated in pure and dry state from the given mixture by the process of sublimation.
CONCLUSION:
The volatile component is separated from the mixture of volatile and non-volatile solid with the process of sublimation.
PRECAUTIONS:
1) The hole on the stem of the funnel should be blocked properly.
2) The outside of the funnel should be wrapped properly with wet filter paper.
3) Wate should not be dropped into mixture
4) The funnel should completely cover the mixture in the porcelain basin.
5) Over heating must be avoided.