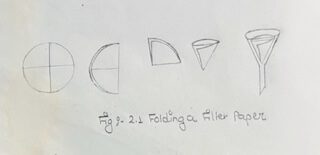
APPARATUS:
1) Beakers
2) Test tubes and test tube stand
3) wire gauze
4) Filter paper
5) Procelain basin
6) Funnel
7) Tripod stand
8) Glass rod
THEORY:
The mixture of sand and common salt, among which one being soluble in water and other being insoluble in water can be separated by dissolutions followed by simple filtration.
Mixture:
A mixture is a combination of two or more substances with out changing their identities. Mixture is divided into two types:
i) Homogeneous mixture
ii) heterogeneous mixture
Residue:
The insoluble component which is left during filtration is called residue
Evaporation:
The process of escaping of liquid from their mixture by continuous heating process is known as evaporation. The purity of sand can be checked with fig NO3 solution and the reaction involved during the purity test is .
NaCL + AgNO3(aq) —-> AgCl + Na NO3
[Curdy white ppt ]
CHEMICALS:
1) Mixture of sand and common salt.
PROCEDURE:
The mixture of sand and common salt was taken in beaker and less than half test tube water was added in it. The mixture was stirred with the glass rod. After then, salt solution in the mixture was stirred with the process of decantation. They separated with the process of decantation. The separated solution was treated with AgNO3 in order to test whether there was preservation of chloride ions or not. When curdy white participation was not observed, the mixture was passed through filter paper placed on the funnel. The sand obtained as residue was Placed on wire gauze and was heated by a burner slightly. The salt solution is further evaporated to separate water and salt.
OBSERVATION:
| S.N. | Experiment | Observation | |
| 1. | The filtrate used to wash. | Curdy white ppt wasobserved. | Sand contains salt. |
| 2. | The sand was wasted several times and sand washed filtrate was again taken in test tube and was tested by AgNO4, | No curdy white ppt was observed. | Sand doesnot combine salt i.e. Cl– is absent. |
TEST REACTION:
NaCl + AgNO3 (aq) ————-> AgCl + NaNO3
(curdy white ppt)
RESULT :
Hence the sand was separated in a pure and dry state from the mixture.
CONCLUSION:
In this way the mixture of insoluble components in water can be separated by filtration in pure and dry state from the mixture of soluble and insoluble solids
PRECAUTIONS:
i) Less than half of the test tube water can be used to dissolve the salt of the mixture.
ii) Apparatus must be handled carefully.
iii) Glass rod should be inclined to the three folded sides of fitter paper in the funnel in order to prevent damaging of filter paper during pouring of sedimented solution.