Introduction:
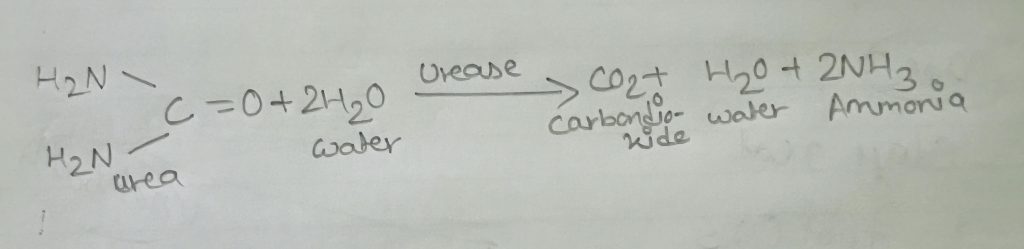
In this test a simple ability of the organism is exploited that is to produce an enzyme called ures so that it attacks the nitrogen and carbon bond in amide compounds such as urea producing end products ammonia, C0₂ and water. The production of urease text can be detected by growing bacteria in a medium containing urea and using a PH indicator such as phenol red. If urea is hydrolysed,ammonia accumulates in the medium and makes it alkaline. This increase in pH causes the indicator to change from orange red to deep pink or purplish red and is a positive test for urea hydrolysis. Failure of a deep pink colour to develop it a negative test.
This text is used to distinguish members of genus Proteus from other enteric non lactose fermenting bacteria (Salmonella, shigella)
Materials required:
i) Urea slant agar or broth for culture
ii) Test/sample organism
iii) Inoculating loop
iv) Incubator
v) Bunsen Burner
Procedure:
i) Prepare a urea broth.
ii) Using aseptic technique inoculate urea tube with the sample (bacterial solution)
iii) Inoculate the tubes for 24 to 18 hours at 37°C.
iv) Examine the broth culture to determine their colour.
v) Note that after incubation, a pink do red constitutes a positive test. But if the original straw colour persists the test is negative
vi) So, based on your observation, determine and record in the report.
OBSERVATION TABLE:
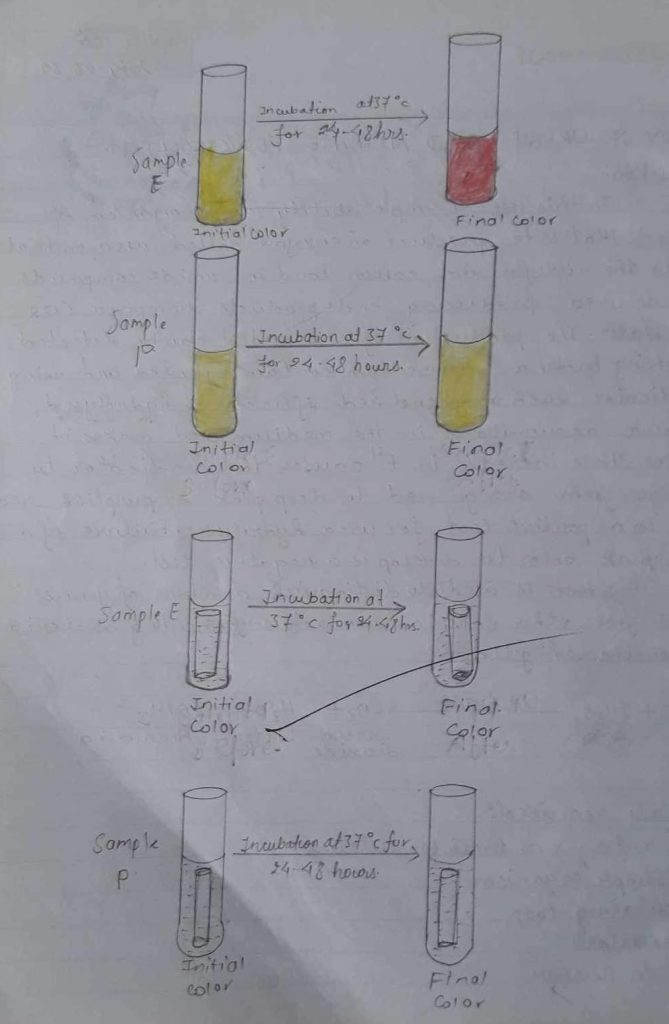
| Organism in broth culture | Indicator used | Initial colour of urea broth | Final colour of urea broth | Remark |
| (E) urease broth | Phenol red | Orange | Pink red | Since pink colour was observed , the given sample is urease +ve. |
| (P) urease broth | Phenol red | Orange | Orange | Since no colour was observed, the given sample is ureas -ve. |
Result:
Deep pink colour was observed so urease positive.
Discussion:
Urease test is performed on urea both containing pH indicator phenolred During incubation, micro-organism possessing urease enzymes will produce NH3 that raises the pH of medium such that phenol red changes from yellow(pH16.8) to a red or deep pink (cerise colour). It means there is a lack of urease enzyme in the test organism.
Precaution:
i) Some bacteria only hydrolyze urea after an incubation period longer than 42 hours.
ii) The urea must not be sterilised in autoclave along with other constituents as it is unstable and
breaks at 15lbs steam pressure. For sterilisation of urea, the filtration method must be urea.
iii) There must not be a need to stab the organism into the butt.
Nitrate Reduction Test:
Introduction:
Many facultative bacteria are able to use the oxygen in nitrate as a hydrogen acceptor in anaerobic respiration, thus converting nitrate to nitrite. This reaction is catalysed by an inducible enzyme called nitrates, also called nitrate reductase. Recall that chemolitho Autotrophic organism i.e., those bacteria that obtain energy through chemical oxidation; they use inorganic compounds as electron donors and CO2 as their primary source and, many chemo organo heterotrophic ie: bacteria that require organic compounds for growth; the organic compounds serve as sources of carbon and energy can use nitrate (No3 -) as a terminal acceptor thus in anaerobic respiration reducing it to nitrite (NO²-). Note that some bacteria can further reduce the nitrite to either the ammonium ion or molecular nitrogen .
nitrate
NO3—————————> NO₂
reductase
KN03———————–> KNO₂ +N2
MATERIALS REQUIRED:
i) Inoculating loop iv) Sample E&P.
ii) Bunsen Burner v) Reagents
iii) Nitrate broth
PRINCIPLE:
When heavy inoculums of the test organism is incubated in a broth containing nitrate then after about four hours, the broth is tested for the reduction of nitrate to nitrite by adding sulfanilic acid reagent. If nitrate has been reduced to nitrite, the acid reagent forms a pink-red compound with α-naphthylamine. When nitrite is not detected it is necessary to test whether the organism has reduced the nitrate beyond nitrite. This test can be done indirectly by checking whether the broth still contains nitrate, Zinc dust is added which will convert any nitrate to nitrite. If no nitrite is detected when the zinc dust is added, it can be assumed that the entire nitrate has been reduced beyond nitrite to nitrogen gas or nitrite reducing organisms.
PROCEDURE:
i) Take a nitrate broth in a test tube.
ii) Inoculate the given sample with the help of a sterile loop in the broth.
iii) Incubate at 37°c for 24 hours.
iv) Add the reagent solution A and solution B into it.
NO₂————–> Pink or red colour
Then look in a durham’s tube.
v) Add a pinch of Zinc dust. If production of pink-coloured organisms is nitrate positive.
OBSERVATION TABLE:
| sample | media | Initial colour of media | Final colour of media | Remarks | |
| P | Nitrate broth | colourless | colourless | N2 | Since N2 is formed the nitrate reduction test is positive. |
| E | Nitrate broth | colourless | colourless | N2 | Since N2 is formed the nitrate reduction test is positive. |
RESULT:
Thus, the nitrate reduction test gives a +ve sample. P gives nitrate reduction test +ve. Broth E ana P sample gave +ve test.
DISCUSSION:
Nitrate reduction test is positive when nitrate is reduced into nitrite which is determined by adding two regents sulfanilic acid and α-pap thylacine Nitrites and Sulfanilic acid react to form a diazonium salt . The diazonium salt then compless with α-naphthylamine to produce a red water soluble azodye. The test will also be positive , when adding sulfanilic acid and α-naphthylamine there is no colour change and by adding zinc power also. There is no change in colour. But if the colour changes into red while adding zinc power the test is negative to the nitrate reduction test and if there is formation of N2 gas in Durham’s tube , the test will be a nitrate reduction test positive.
CONCLUSION:
Hence, In this way by adding different reagents in the given media, urease test and nitrate reduction test was done in the laboratory.
PRECAUTIONS:
i) Durham’s tube should be kept in an inverted position in the test tube.
ii) The colour of the media should be observed carefully.
iii) In a nitrate reduction test, sulfanilic acid should be added before α-naphthylamine.
REFERENCES:
Manandhar.S, Shama S (2006), Practical Approach to Microbiology , 3rd edition, National Book Centre, Bhotahity, Kathmandu Page no. 82-83.


