Introduction:
The main aim of this experiment is to distinguish between vegetative cells and endospores.
Members of anaerobic genera Clostridium and Desurfotomecule and the aerobic genus Bacillus are examples of organisms that have the capacity to exist either as metabolically active vegetative cells or as highly resistants metabolically inactive call types called spores. When environmental conditions become unfavourable for continuing vegetative cellular activities, particularly with the exhaustion of a nutritional carbon source, these cells have the capacity to undergo sporogenesis and give rise to a new intracellular structure called the endospores which is surrounded by an impervious layer called spore coats. Spore position in the mother Cells or sporangium frequently differs among species making it of considerable value in identification. Spores may be centrally located, close to one end (sub-terminal) or definitely terminal.
Primary stain:
Malachite greens: Malachite green is used first but because of its impervious coats bacterial spores will not accept the primary stain easily. The application of heat is required for further penetration. After the primary stain is applied and the smear is heated, both the vegetative cells and spore will appear green.
Decolorizing Agent (Distilled Water)
Once the spore accepts the melachite green it can not be decolorized by water which removes only excess primary stain. The spore will remain green. It can not be decolorized by water which removes only the excess primary stain. The spore will remain green. On the other hand, the stain does not demonstrate a Strong offinity for vegetative cell components and the water removes the malachite green. So, the vegetative cell becomes colourless.
Counter Stain:
Sat Canin: The contrasting red stain is used as the second reagent to colour the decolourized vegetative cells, which will beat the centre stain and appear red. The spores retain the green colour of the primary Stain.
Materials required:
Apparatus: Bunsen Burner, Hot plate, staining tray, Inoculating loop. Glass slides, Bibulous paper, Lens paper and microscope.
Reagents: Malachite green. distilled water and Safranin culture.
Culture: 48 to 72 hours nutrient agar start culture.
Procedure:
i) A clean grease free slide was taken.
ii) Athin smear was made by sterile technique.
iii) Smear was allowed to air dry and heat fixed.
iv) Smear was flooded with malachite green and steamed, Over boiling water for 10-15 minutes and was added, Addition malachite green if it boils off.
v) Slide was removed from boiling water and was cooled and was washed with water.
vi) Counterstain safranin was treated with a smear for minutes and was washed and distilled with water.
vii) It was dried and was observed under the oil immersion lens of a microscope.
Observation table:
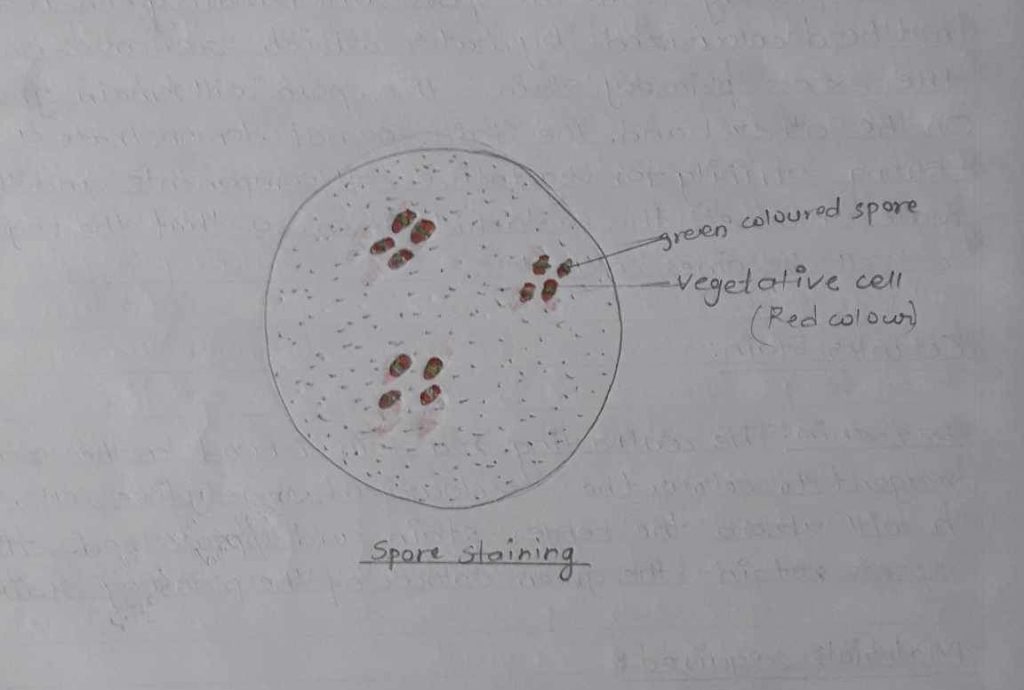
| Staining method | Stain Used | Colour of organism | Colour ofspore | Located ofspore | Result |
| Spore staining | Malachitegreen Safranin | Red | Green | Centrally Located | The spore was located at the centre and green in color |
Result:
The organisms was seen rod shaped, red in colour, and green colour spore was seen.
Discussion:
Under certain unfavourable conditions, it gives rise to endospore and vegetative cells of red in colour and the spore was green in colour. The spore was located at the central. Thus we concluded that it was rod shaped cas well.
Conclusion:
Spore staining was studied and performed to distinguish the vegetative cell and endospore.
Precaution:
i) We must not allow primarily stains to evaporate during boiling over water baths.
ii) Heat fixation should be done otherwise bacteria would not be seen.
Reference:
Shah PK, Dahal PR.& Amatya J (2009) Practical microbiology (Revised edition), Delta offset press, thapathali, Page no. 46-49.


