Introduction:
The biochemical reactions of the microorganisms are very much important in species identification. Since all physiological reactions in organisms are catalysed by enzymes and since each enzyme is produced by individual genes, the detection of enzymes produced is the special tool for species identification. Various biochemical tests are Catalase and Oxidase tests.
Catalase Test:
This test is used to determine the presence of Catalase as an enzyme in microorganisms that decomposes hydrogen peroxide to release oxygen. Catalase is a hemoprotein, prosthetic group that is made up of four atoms of trivalent iron (Fe³+) per molecule which retains its oxidised state during enzyme activity. And as oxidative end product of aerobic breakdown of sugar, hydrogen peroxide is formed. Thus, reduced flavoprotein reacts directly with gaseous oxygen by way of electron reduction to form hydrogen peroxide and not by direct action between hydrogen and molecular oxygen.
F PH₂ +0₂ ———————————-> FP+H₂O₂
(reduced flavoprotein) (oxidised Flavoprotein)
If H₂O₂ is allowed to accumulate death of bacteria occur in presence of oxygen due to suicidal act of H₂O₂ production in absence of catalase production. In decomposition of H₂O₂, one molecule acts as the substrate and other as donor, substrate reduced by Hydrogen atom supplied by donor, resulting in a reduced substrate H₂O & oxidised donor O₂.
catalase
2 H₂O₂ ——————->2 H₂0+0₂
reduced(donor) oxidised (donor)
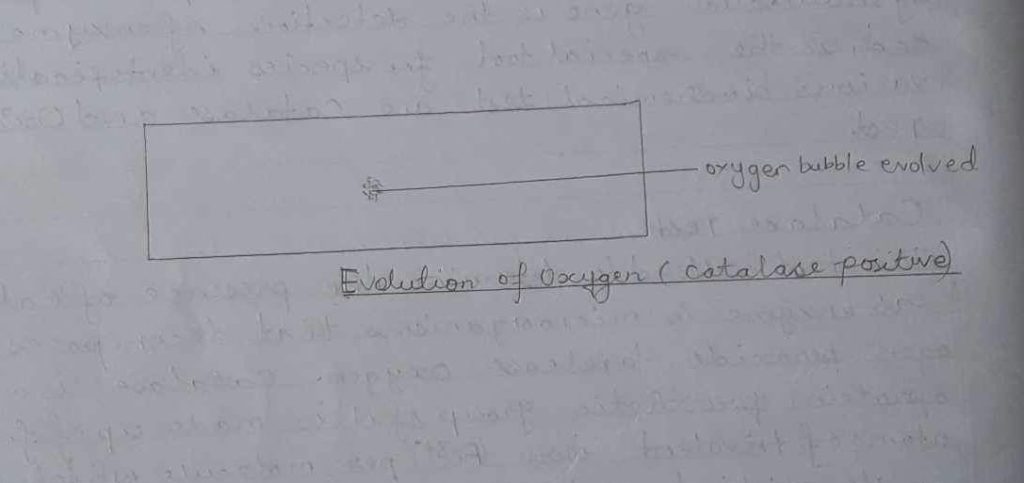
So, evolution of oxygen bubbles occurs in case of catalase positive organisms.
Material Required:
i) Glass rod
ii) Glass slide
iii) Bunsen burner
iv) Pure plate culture organism
v) Hydrogen peroxide
vi) Pipette
Procedure:
i) A clear grease free glass slide was taken in which one loopful of organisms from 18-24 hours a pure colony was transferred in the centre of the slider.
ii) With the help of a pasteur pipette, one drop of 3% H₂O₂ was added over the organism on the slide.
iii) Thus effervescence of gas bubble was observed.
Observation Table:
| Sample No. | Reagent used | Observation | Inference |
| A | Hydrogen Peroxide | Effervescence of gas bubble | Catalase + ve |
| B | Hydrogen Peroxide | Effervescence of gas bubble | Catalase + ve |
Result:
Thus, there was evolution of the gas bubble with 2 to second due to oxidation of organisms producing water & oxygen and thus the given organism was catalase positive.
Discussion:
The given tested organism was catalase positive because it could convert hydrogen peroxide into water and oxygen. Due to the presence of an enzyme catalase in microorganisms H₂02 was converted into water and oxygen. Hence, the test organisms gave positive heat.
Conclusion:
Hence, the organism tested was catalase positive as there was evolution of oxygen.
Precautions:
i) Tested organisms should not be taken from blood culture. RBC contains catalase and their presence will give a false positive result.
ii) Growth for catalase test must be from 18-24 hours culture.
iii) H₂O₂ must be fairly fresh as it is very unstable and easily breaks down on exposure to light.
Oxidase Test:
This test is used to determine the presence of oxidase enzymes in bacteria that will catalyse the transport of electrons between electron donors in bacteria and redox dye tetra-P-phenylenediamine. The oxidase reaction is due to the presence of cytochrome by molecular Oxygen, which in turn acts as an electron acceptor in the terminal stage of ETS. Molecular oxygen oxidises a substrate through intervention of ETS during respiration.
All oxidase positive organisms are either aerobic or facultative anaerobic with exception of vibrio species which requires microaerophilic oxygen. Positive Oxidase reaction is also limited to those organism capable of growing in presence of oxygen and at some time producing enzyme catalase. Obligate anaerobes lack oxidase activity since they are unable to live in atmospheric oxygen & do not possess a cytochrome oxidase System. An oxidase reagent oxidises the reagent to form a coloured compound, Indophenol.
Materials Required:
i) Glass Rod
ii) Spirit lamp
iii) Glass slide
iv) Filter Paper strips soaked in 1% tetramethyl-P-phenylene diamine dihydrochloride.
v) Fresh sample of different bacteria.
Procedure:
i) Filter paper was placed in the centre of the clean glass slide.
Filter paper was soaked in 1% tetramethyl-p-phenylenediamine dihydrochloride.
ii) With a glass rod, a colony of a test organism was taken and it was smeared on filter paper.
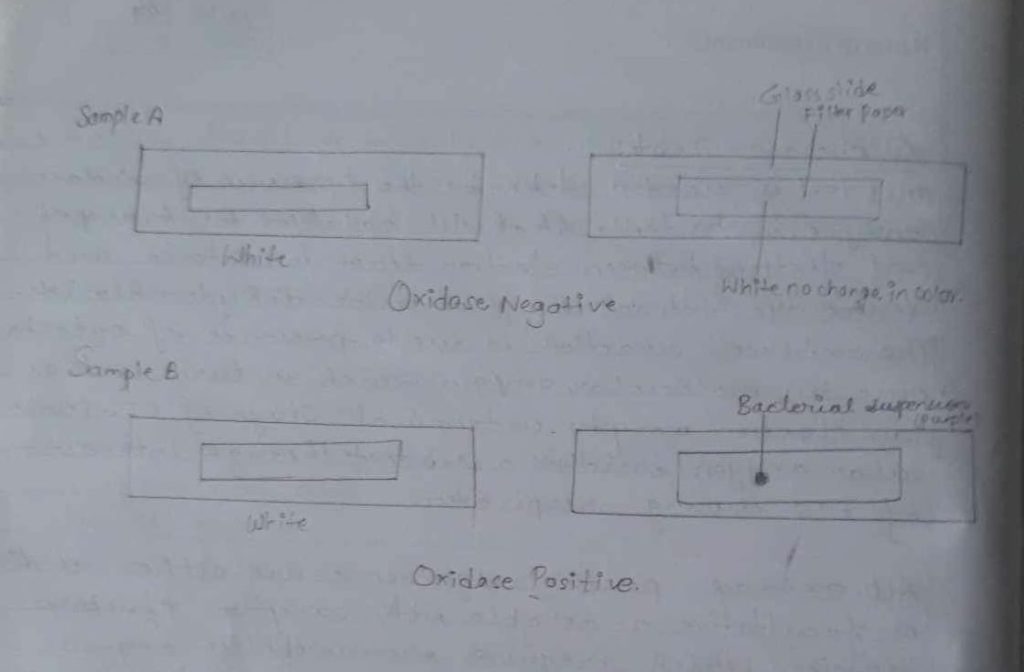
iii) Then the colour development took place which was blue purple colour observed within 5-10 seconds.
Observation table:
| Sample No. | Reagent used | Colour of filter Paper Initial Color | Colour of filter Paper Final Colour | |
| 1 | 1%. tetramethyl-p-Phenylenediamine dihydrochloride | White | colourless. | Oxidase-ve |
| 2 | 1%. tetramethyl-p-Phenylenediamine dihydrochloride | White | Purple | Oxidase +ve |
Result:
Thus, an oxidase test was done and only sample B was confirmed as oxidase positive which was changed in purple colour and sample A remained as it was i.e no. change in colour of filter paper which showed negative oxidase..
Discussion:
It was confirmed that among tested organisms, Organisms of sample. B was oxidase +ve because the active part of dye i.e. paraphenylene. combined with oxygen in presence of oxidase cytochrome to form a complex indophenol, was a purple coloured and this was due to the presence of such complex compounds. And sample ‘A’ organism was oxidase negative as the active part of the dye cannot combine with oxygen in presence of oxidase cytochrome to form a complex indophenol.
Conclusion:
Hence, oxidase test was performed with various micro-organisms among which the micro-organisms, a sample ‘B’was oxidase positive as its colour charged to purple & other sample ‘A’ could not change the colour was oxidase negative.
Precaution:
i) Acidity inhibit oxidase enzyme activity So, oxidase test should not be performed on any colonies growing on medium containing glucose, otherwise false negative test in any result.
ii) Filter Paper should not be kept in a wet place.
iii) Inoculating metal loops should not be used.
References:
Shah PK, Amalya J, Dahal P.R. (2013) “Practical Microbiology” Delta Offset press, Thapathali Kathmandu Page No. 118.125.

