REQUIREMENTS:
Methyl salicylate: 5 ml of NaOH solution (4N) = 40m Conc. HCL.
THEORY:
The ester methyl salicylate when refluxed with caustic soda solution i.e. NaOH solution acidified with dilute acid methyl alcohol & Salicylic acid is formed. This reaction is called hydrolysis of esters.
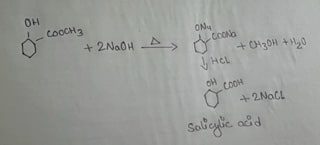
PROCEDURE:
5 ML of Methyl salicylate & 40 ml of N NaOH had been taken in a 250ml conical flask fitted with an air condenser. The mixture was then heated & refluxed until no oily drops got floated on the surface of the liquid. The flask was cooled & concentration hydrochloric acid i.e. HCL should be added slowly till the mixture was acidic where whites precipitate of salicylic acid formed. In the similar way the solution of mixture with white precipitate of salicylic acid was filtered at the pump. The ppt was washed with water & then dissolved the salicylic acid in a little volume of water by bailing. A pinch of bone charcoal was added during boiling in which a hot solution
was filtered through a butcher’s needles on cooling the filtrate. The acid was collected by function filtration and washed it with a little cold water & finally dried between
the folds of a filter paper.
RESULT:
Appearance- colourless needle like crystals.
yields – 4gm
M.P – 1560C
CONCLUSION:
Hence, the crystals of the salicylic acid by the hydrolysis of Methyl salicylate are prepared.
