APPARATUS REQUIRED:
1) Salicylic and
2) Test tube
3) Conical flask
4) Charcoal zn etc.
THEORY:
When salicylic acid is added with acetic acid (CH3COOH) in the presence of zinc (2n) then aspirin is obtained. This reaction is called acetylation of salicylic acid.
Reaction:
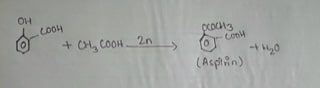
PROCEDURE:
Some porcelain chips in the 250 ml were dissolved into conical flasks. The mixture was refluxed by using a reflux condenser. It was taken in conical Flask & Condensed for about 30 minutes. And the mixture was filtered with the help of filter paper as well as the mixture at 100 ml of cold water was poured which contained ice drops.
The amount of Aspirin purified by recrystallization. Crude sample was dissolved in 50% acetic acid with some bone Charcoal. The mixture was boiled and the hot solution was filtered. The filtrate was then cooled & the crystal was collected in the buchner funnel.
RESULT:
Appearance: colourless needle like crystal
Yield: 4g
Melting point. 135-136°C
CONCLUSION:
Hence, the crystals of the aspirin by the acetylation of salicylic acid were prepared.
