THEORY:
Modern inorganic chemistry has been described as the study of preparation, qualitative and quantitative composition, structure and reaction and uses of chemical substances most of which does not contain carbon; an ingot salt is formed by the interaction of a base or metallic oxide or metal as base.
Qualitative analysis concerns the kidner constituent present in the substance. The basis of qualitative organic analysis is the properties of ions present in salt.
Analysis is done in dry and wet ways:
1) Dry test:
In a dry way, characteristic properties of a substance when subjected to acids, alkali, oxidizing and reducing agents; generally with reference to its color; odor state are basic of analysis. Dry that is performed before a wet test.
2) Wet way test:
In wet way tests, radicals are detected in solution by their ionic reactions. Acid radicals are identified by their reaction with particular reagents. Metal ion are classified into definite analysis groups basic for the classification of metal ions group is the reactive stability of their chloride sulfide hydroxides and carbonates in various media and their solubility product. In a particular group, the ions are separated from the preposition with a particular reagent, and confirmed by characteristic perspective or Colour of solution. In the mixture of two or more inorganic salt. It is however not always possible to identify the respective ions and an Individual salt but the various cations and anions present in mixture can always be detected.
PHYSICAL PROPERTIES:
sample: S1
State: Solid
Color: Bluish and white
Odor: Odorless
1) Dry test for acid Radicals:
A) Action of conc. H2SO4.
| Experiment | Observation | Inference |
| 0.2 g of substance was taken in test tube with 2-3 ml of conc.H2SO4 and warmed gently. | colorless gas evolved with a pungent odor which formed in air and produced white fumes with a glass rod moisturized with conc.NH3 solution Cl2 gas evolved in addition to MnO2. | HCl gas from chlorines. |
2) Wet test for acid Radicals:
| Experiment | Observation | Inference |
| 1) AgNO3 Test: | ||
| 1 ml of salt solution in a test tube added with 1 ml of dil.HNO3 along with a few drops of AgNO3 solution. | White ppt. Dissolved in NH3 solution and reappeared when acidified by dil. HNO3. | Presence of Cl– . |
| 2) BaCl2 Test: | ||
| 1 ml of salt solution in a test tube and BaCl2 solution was added. | White ppt. insoluble in dil. HNO3. | Presence of S04— . |
REACTION INVOLVED:
1) Dry test for acid radicals:
a) Test with conc.H2SO4
Halides with conc.H2SO4 on heating liberate hydrogen halides gas:
gently heating
NaCl + H2SO4 ——————–> NaHSO4 +HCl ↑
strong testing
2NaCl + H2SO4 ——————-> Na2SO4 + 3HCl ↑
Halides salts on heating with conc.H2SO4 in presence of MnO2 elementary halogens gas are liberated which is identified from their color.
2NaCl + 3H2SO4 + MnO2 ——–> 2NaHSO4 + MnSO4 + 2H2O + Cl ↑
2) Wet test for acid radicals:
a) Reaction of Cl–
i) With the AgNO3 solution.
White curdy ppt. of silver chloride, insoluble in dil. HNO3 but soluble in dil. NH3 solution and in sodium thiosulphate solution.
NaCl + AgNO3 ———–> AgCl + NaNO3
AgCl + 2NH3 ————> [Ag(NH3)2] Cl
[Ag(NH3)2] Cl + 2HNO3————> AgCl + 2NH4NO3b) Reaction for SO4—
i) with BaCl2 solution:
white ppt. of barium sulfate Insoluble in dil mineral acid.
ii) Na2SO4 + BaCl2 ———-> BaSO4 + 2NaCL
iii) With AgNO3 solution:.
White crystalline ppt. of silver sulfate with conc. solution
Na2SO4 + 2AgNO3 ———-> Ag2SO4 + 2NaNO3
3) Day test for Basic Radicals:
a) Action of heat
| Experiment | Observation | Inference |
| 0.1 gm of the given sample was heated in a dry test tube. | Colour changed from green to brown on strong heating. Also white sublimate was formed. | Cl– salt . May be NH4+ ,Ag, As or Sb salt. |
b) Test for NH4– radical
| Experiment | Observation | Inference |
| i) Small amount of sample was heated in a test tube with NaOH solution. | I) A gas having smell of ammonia evolved which | Presence of NH4+ salt. |
| a) Turned moist red litmus to blue. | ||
| b) Produced dense fumes with conc. HCL on glass rod. | ||
| c) Mode mercurous nitrate paper black. |
4) Wet test for Basic Radicals:
a) Preparation of solution:
About 19 m of salt mixture (S1) was dissolved in a 100 ml beaker and prepared. Solution was taken for systemic group separation of cations as:
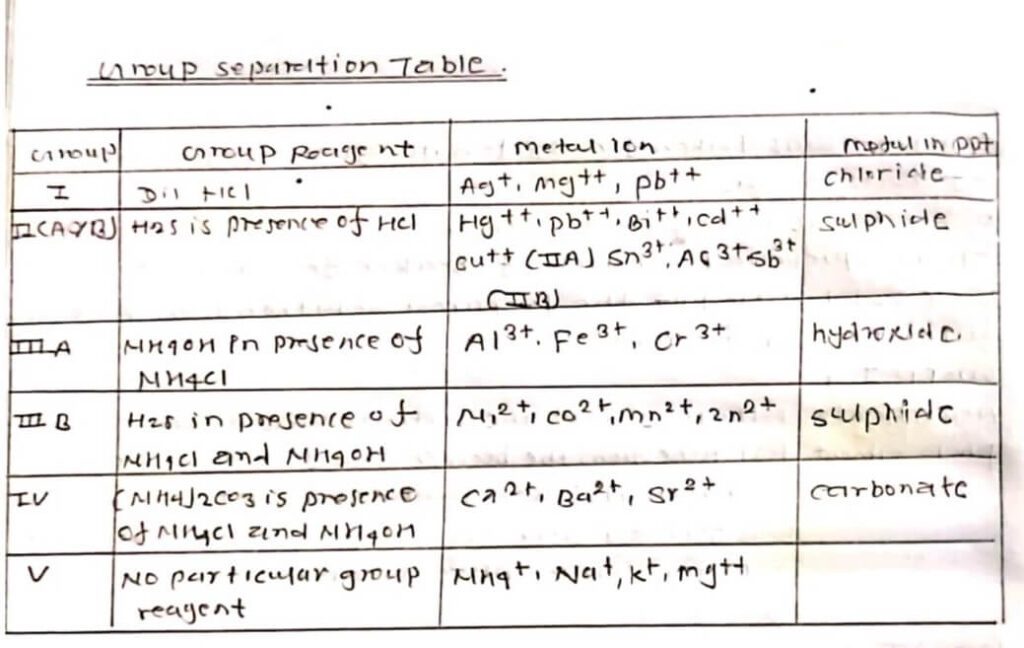
b) Systemic Group separation:
To the prepared solution of the beaker a few drops of HCl were added. No ppt the prepared solution was proceed further as a filtrate:
Group (I)
| AgCl; HgCl2 pbcl2 absent | Filtrate: 1ml of the filtrate was taken in the test tube from the beaker and a few drops of HCl and then Has was bubbled in hot solution ppt was formed and Has was passed to the whole hot Solution till complete precipitation. |
Group (II)
| CuS ; PbS, HgS present | Ppt was transferred into a porcelain basin and 5 ml of dil HNO3 was added to the whole solution and boiled for a few minutes. Residue was dissolved and filtered. was proceeded further as: |
| Filtrate: A small protein was tested with dil.H2SO4 alcohol, a white ppt. indicated pb, concentrated in the fume chamber until chick, white fumes appeared. It was cooled, and diluted with 10 ml water and filtered. The filtented ww proceeded further as: | |
| Filtrite: NH3 solution was added in excess and filtered again the filtrate was preceded the filtered was divided into two unequal parts.a) To smaller protein, potassium ferrocyanide and acetic acid was added: Reddish brown ppt formed – Cu++ present. |
REACTION INVOLVED:
1) Dry test for Basic Radicals:
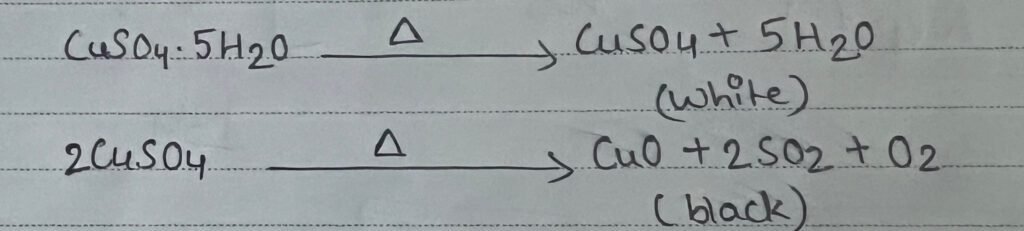
a) Change in color of salt: Certain inorganic salt decompose to their oxide of anhydrous form accompanied by change in color salt of metals like Cu, Fe, Ni, Bi, Pb, mn, Cd, Cr, Hg change their color on heating coloured salt of Cu, Mn, Ni, Co gives black oxide.
2) Wet test for Basic Radicals:
Sulfides of group (IIA) are insoluble in yellow ammonium Sulfide whereas sulfides of group (IIB)dissolves in yellow ammonia sulfide forming their salt.s In this way group (IIA) and (IIB) are separated:
CuS + 8HNO3 ————> 3Cu(NO3)2 + 2NO +3S + 4H2O
CuSO4 + 4NH4OH ———-> Cu(NH3)4SO4 + 4H2O
2CuSO4 + Ku[Fe(CN)6] ——-> Cu[Fe(CN)6]2 + 2K2SO4
RESULT:
Hence, the acid radicals were found to be Cl– and SO4 – and basic radicals were found to be MH4+ and Cu ++.
CONCLUSION:
Hence, the complete Analysis of the given salt mixture was done by dry and wet ways.
PRECAUTION:
i) Accurate amount of samples should be taken.
ii) Color change and gas evolved should be sharply noted.
iii) Apparatus, and chemicals should be handled carefully.
