APPARATUS REQUIRED:
a) Conical flask
b) Filter papers
c) Test tube
d) Funnel
e) Beaker
CHEMICALS REQUIRED:
a) Aniline
b) Benzoyl chloride
c) 10% Sodium hydroxide
THEORY:
Benzonilide is prepared in the lab by the benzoylation of aniline where aniline is treated with benzoyl chloride in presence of sodium hydroxide. Replacement of H-atom of NH2 group of aniline by benzoyl group (C6H5CO) takes place. Hence the process is known as benzoylation.
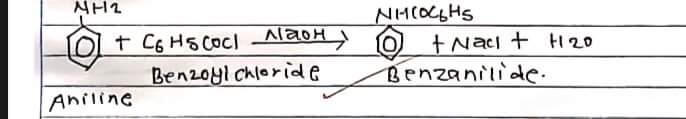
unreacted benzoyl chloride reacts with NaoH forming auto benzoate and remains in solution.
PROCEDURE:
8 ml of Aniline, 50 ml of 10% sodium hydroxide (NaOH) solution and 7 ml benzoyl chloride solution was taken in a 250 ml conical flask. The flask was then shaked cooled vigorously till the smell of benzyl disappeared which required generally 10-15 minutes. The content was diluted with the cold water and frittered off the precipitated product. Then the product was washed with cold water and was recrystallized from cold alcohol/water.
RESULT:
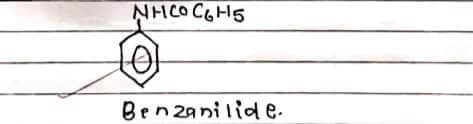
Benzemilide appeared with white crystalline with melting point 162°C.
CONCLUSION:
Hence, the benzanilide was prepared in the laboratory by the process of benzoylation of aniline.
PRECAUTIONS:
1) Benzoyl chloride should be added carefully.
2) Apparatus should be handled carefully
3) Conical flask should be Shaken vigorously white cooling.
STRUCTURE: