MATERIALS REQUIRED:
a) Methyl Salicylate
b) NaoH solution
c) Conc. HCI
THEORY:
The ester methyl Salicylate when treated with caustic Soda i.e. NaOH solution acidified with dilute and methyl alcohol and salicylic acid is formed. This reaction & called hydrolysis of ester.
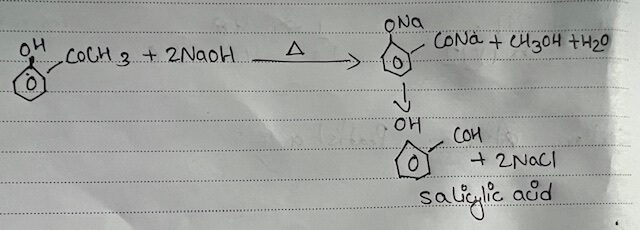
5 ml of methyl salicylate and 40 ml of dil.NaoH was taken in a 250 ml conical flask with air Condenser. The mixture was then heated and refluxed until no oily got floated on the surface of the liquid. The float was cooked and Conc. HCI was added slowly till mixture was acidic where white pet-of salicylic acid was formed. In a similar way, a solution of mixture with white ppt of salicylic acid was filtered at the pump. The ppt was waned with water and then dissolve the salicylic pump acid in a little volume of water by boiling A pinch of bone charcoal was added during boiling in which hot solution was filtered through a bunches when Salicylic acid would crystallize out as colorless needles on waiting the filtrate. The acid was collected by suction of filtration and washed with a little cold water and finally dried between the folds of filtre paper.
RESULT:
The colorless needles like crystals were obtained about 4 gm in the filter paper.
CONCLUSION:
Thus the crystals of the salicylic acid by the hydrolysis of methyl Salicylate was prepared.
PRECAUTIONS:
1) Apparatus must be heated carefully.
2) The mixture should not evaporate so the pipe must be wet.
