MATERIALS REQUIRED:
a) test tube
b) test tube holder
c) Funnel
d) Beaker.
CHEMICALS REQUIRED:
a) Dimethyl aniline
b) Sodium carbonate
c) Sodium nitrate
d) Sulphuric acid
e) Conc. HCI
THEORY:
Methyl orange is a light orange coloured solid soluble in alcohol. It is an important Indicator in acid-base solution titration. It is prepared by diazotization of sulphonic acid followed by coupling with dimethyl aniline in presence of acid at ice cold temperature.
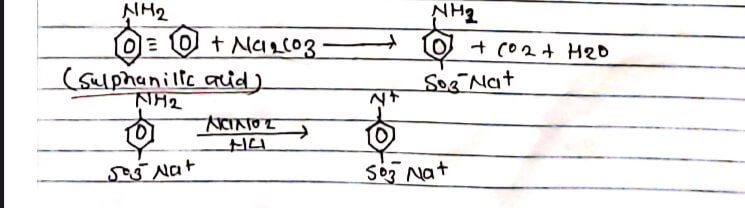
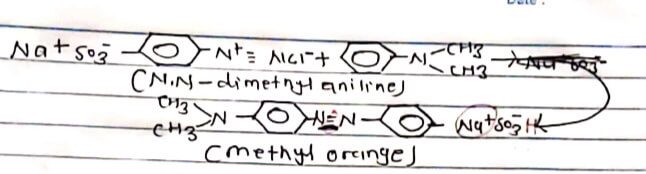
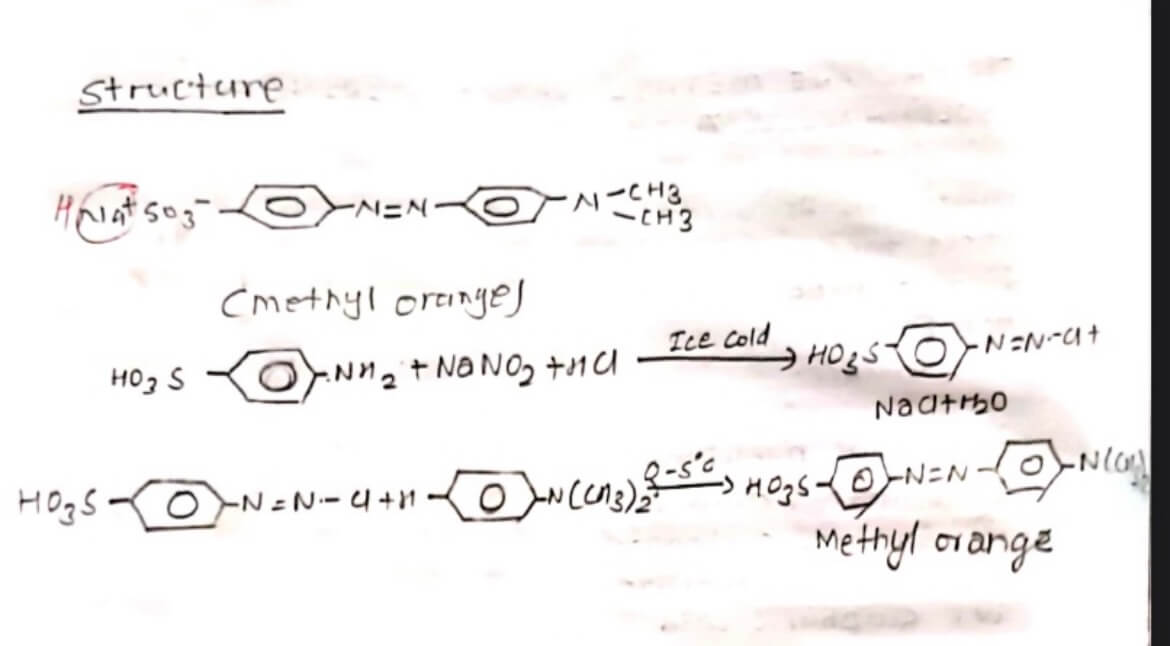
PROCEDURE:
0.5 gm of Na2CO3 was dissolved in 20 ml H2O and 29m Sulphanilic acid was added in a beaker and was immersed in cold water. In a test tube 19m of NaNO2 was dissolved in 2 ml H2O. Then the solution in the beaker and the test tube was mixed slowly and concentrated conc. HCI was added to the mixture. Now, 1 ml dimethyl aniline 1ml galactic acetic acid and 5-10 ml NaOH was slowly added. Then, the resulting solution was filtered. The residue was filtered with cold water, and was dried-Light orange crystal methyl orange obtained.
RESULT:
The light orange crystals of methyl orange were prepared from Sulphanilic acid.
CONCLUSION:
Thus, by this experiment methyl oranges can be prepared in the laboratory from sulphanilic acid.
PRECAUTIONS:
1) Apparatus should be handled carefully.
2) Chemicals should be handled carefully.
