MATERIALS REQUIRED:
a) Conical flask
b) Stand
c) Beaker
CHEMICALS REQUIRED:
a) salicylic acid-3gm
b) zinc dust -5gm
c) charcoal.
THEORY:
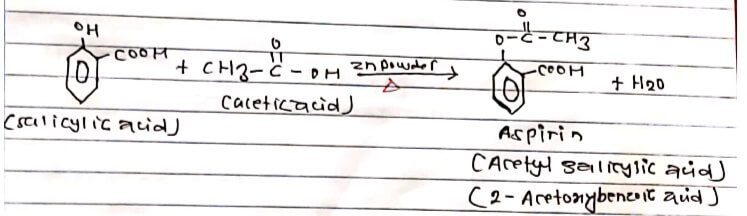
Aspirin is prepared in the lab by heating salicylic acid with acetic acid in presence of a small amount of zinc powder at 800C on water bath replacement of H-atom-of-OH group by acetyl group (-COCH3) takes place. Hence the process is known as acetylation.
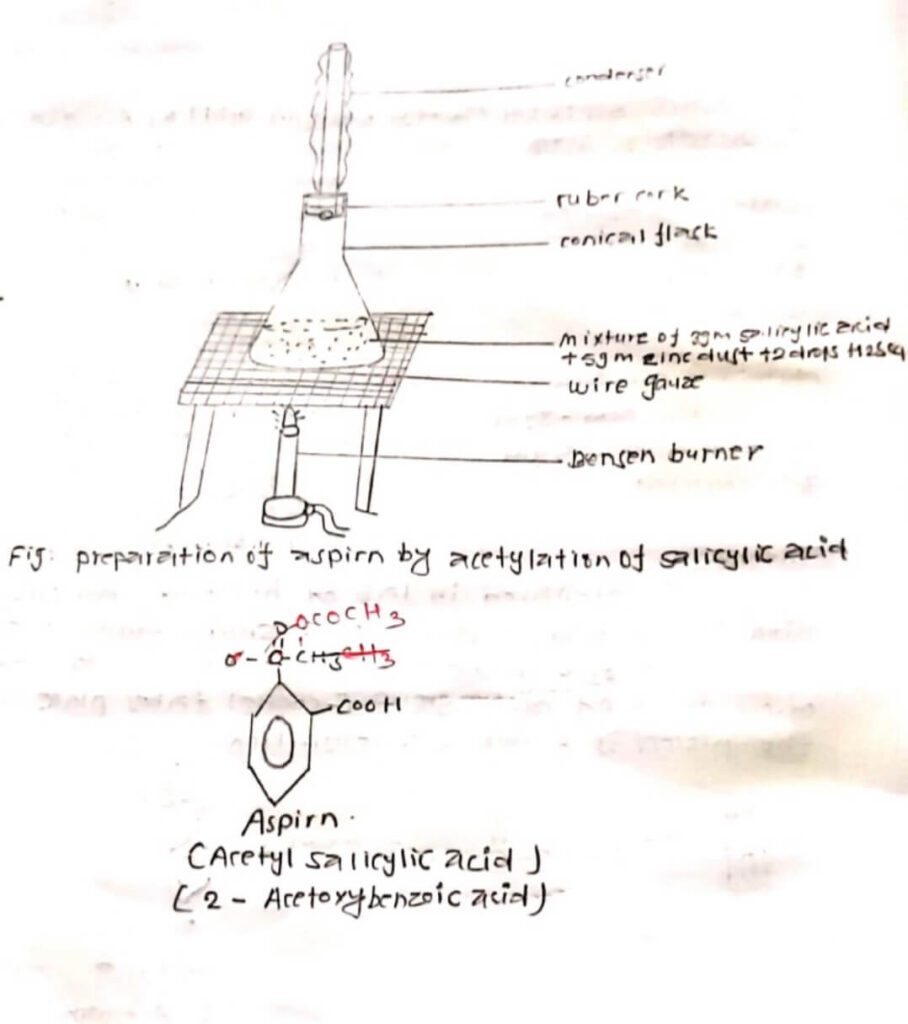
PROCEDURE:
A mixture of 3gm salicylic acid and 5 gm zinc dust with 105 ml of acetic acid was taken in a 150 ml conical flask filled with air condenser. Then it was refluxed for about 40 minutes. It was allowed to cool initially and then the whole solution was poured in cold water. Aspirin separated out as a white crystalline substance. Small amount of charcoal was added after filtration. The mixture was heated again and filtered again in which aspirin separated out as white crystalline material.
purification and Crystallization:
The sample was dissolved in the minimum quantity of 50% acetic acid and by slight heating. The hot solution was filtered rapidly under suction. Then the filtered was cooled and the crystals should be filtered on a Burner funnel with the help of a suction pump. The crystals were dried by pressing them gently between the folds of filter paper.
RESULT:
The colorless needle Shaped crystals were obtained.
CONCLUSION:
Hence, the crystals of aspirin can be obtained by acetylation of salicylic acid.
PRECAUTION:
1) Filtration must be done properly.
2) Apparatus Should be handled carefully.
3) Condenser must be used properly.
