APPARATUS REQUIRED:
i) Woulf’s bottle
ii) Thistle funnel
iii) Delivery tube
iv) Corks
v) Gas Jar
vi) Test tube
CHEMICAL REQUIRED
i) FeS (Ferrous Sulphide)
ii) Dilute sulphuric acid (dil. H₂SO4)
THEORY
Hydrogen sulphide gas (H₂S) is prepared in the laboratory by the action of dil. H₂SO4 or dil. HCl on ion sulphide (FeS) as:
Fes +dil. H₂SO4 ————–> FeSO4 + H₂S ↑
Fes + dil. 2HCl —————-> FeCl2 + H₂S ↑
Ionic reaction: FeS + 2H+ ———–> Fe++ + H₂S ↑
HNO3 of any concentration and conc. H₂ SO4 cannot be used as they are a good oxidizing agent oxidized H₂S so formed to sulphur.
3 H2S+ dil. 2HNO3 —————> 4H₂O+2NO +3S
H₂S + Conc. 2HNO3 ————–> 2H₂O+2NO₂ +S
H₂S + Conc. H₂SO4 —————>2H₂O +SO₂ +S
The gas being heavier than air and slightly soluble in water and is collected in a gas jar by upward displacement of air.
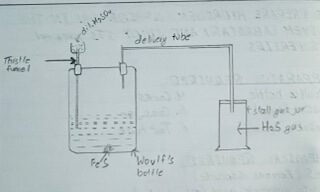
PROCEDURE
Small pieces of iron sulphide (FeS) were taken in woulfe’s bottle and filled with thistle funnel and delivery tube as shown in figure. Dil. H₂SO4 was poured from thistle funnel as the reaction occurs, the gas so produced was passed through delivery tube and finally collected into chemical reagents in the test tube for studying its properties as :-
OBSERVATION TABLE
| S.N. | Experiment | Observation | Inference |
| 1. | Colour and odor of the gas was noted. | The gas was colorless but rotten egg odor was observed. | The H₂S gas is colorless with rotten egg smell. |
| 2. | The moist red and blue litmus papers were introduced into the gas jar filled with H₂S gas. | The blue litmus paper was turned into red litmus paper and remained unchanged. | H₂S gas acidic in nature. |
| 3. | Burning match stick was introduced into the gas jar filled with H₂S gas . | Burning match stick was extinguished & gas was burnt with pale yellow flames. | H₂S gas was non- supporter but is combustible. |
| 4. | In a test tube filled with H₂S gas was inverted over water in the water trough. | The water level was raised slowly inside the gas jar. | The H₂S gas is slightly soluble in water. |
H₂S gas as a reducing agent:
| 5. | H₂S gas was passed over wet filter paper with lead acetate solution (lead acetate paper). | The wet filter paper turned into black. | Lead sulphide(PbS) is formed. |
| 6. | H₂S gas was passed through an acidified KMnO4 solution. | The pink color of KMnO4 sol” was discharged to colorlessness. | The acidified KMnO4 solution is reduced but H₂S was oxidized. |
| 7. | H₂S gas was passed through an acidified K₂ Cr₂O7 solution. | The orange color of K₂ Cr₂ O7 was changed into green watercolor. | The acidified K₂ Cr ₂ O7 solution is reduced but H₂S gas was oxidized. |
| 8. | H₂S gas was passed through the FeCl3 solution. | The reddish brown color of Fells was changed into pale yellow turbidity. | The Has gas reduced ferric and ferrous salt. |
H2S gas an analytical reagent:
| 9. | H₂S gas was passed through an acidified CuSO4 solution. | Black ppt. was observed. | Cupric sulphide(CuS) is formed. |
| 10. | H₂S gas was passed through an alkaline CuSO4 solution. | Black ppt was obtained. | Cupric sulphide was formed. |
| 11. | H₂S gas was passed through an acidified NiSO4 solution. | No ppt was obtained. | H₂S gas cannot be reduced NiSO4 in acidic solution. |
| 12. | H₂S gas was passed through alkaline NiSO4 solution. | Black ppt was obtained. | Nickel sulphide was formed. |
| H₂S gas was passed through acidified ZnSO4 solution. | No ppt was observed. | H₂S gas cannot reduce acidified ZnSO4 solution. | |
| H₂S gas was passed through an alkaline ZnSO4 solution. | white ppt was formed. | Zinc sulphide (Zn S) is formed. |
REACTION INVOLVED:
burning
2H₂S ————–> 2H₂O +2S ↓
(CH3COO)2 Pb ———–> Pbs ↓+ 2CH3 COOH
(lead sulphite)
(Black ppt)
2KMnO4 + 3H2SO4 + 5H₂S ———> K₂ SO4 + 2MnSO4 + 8H₂O + 5S
(Colourless)
K2Cr₂O7 + 4H₂SO4 + 3H₂S ——–> K₂SO4 + Cr₂ (SO4)3 +7H₂0 + 3S
(green colour)
2 FeCl3 + H₂S ————> FeCl₂ +S + 2HCl
(Reddish brown) ( pale yellow)
Acidic
Cu++ + H₂S —————-> CuS↓ + 2H+
(black ppt)
Alkaline
Cu++ + H2S —————–> CuS + 2H+
(black ppt)
Acidic
Ni++ + H₂S ——————-> No reaction
Basic
Ni++ + H₂S ——————–> NiS↓ + 2H+
(black ppt)
Acidic
Zn++ + H₂S ——————-> No reaction
Basic
Zn++ + H₂S ——————–> ZnS↓ + 2H+
(White ppt)
RESULT
H₂S gas was prepared by treating FeS and dil HCL and studied its properties.
CONCLUSION
H₂S gas can be prepared by treating ferrous sulphide with dil HCL.
PRECAUTIONS
1. All the apparatus should be handled carefully.
2. The cork should be fitted tightly.
3. The lower end of the thistle should be dipping into the acid.
4. The edge of the delivery tube should not touch the solution.
