APPARATUS REQUIRED
i) Porcelain basin
ii) Burner, tripod stand and wire gauze
iii) Test tube
iv) Sand bath
v) Filter paper
vi) Test tube stand
CHEMICAL REQUIRED:
i) Bench NaOH
ii) Bench HCL
iii) Phenolphthalein
THEORY
Neutralization reaction:-
The reaction in which acid reacts with base to give salt and water.
NaOH + HCL ————> NaCl + H2O
(Base) (Acid) (Salt) (Water)
Neutral point:-
The point at which acid completely neutralizes with base.
Indicator:
The chemical substance which indicates the neutral point by changing color.
Crystals:
The solid having particular geometrical shape, sharp edge shining appearance and having sharp melting point is known as crystal.
Evaporation:
The process of conversion of a liquid into its vapor form.
Evaporation of Salt:
As the water evaporates, the salt doesn’t leave with it. Therefore, the concentration of salt in the water left behind increases. Eventually, the concentration gets too high that the water becomes supersaturated and the salt will begin to re-crystalline into a solid. When all the water is gone, you will have salt.
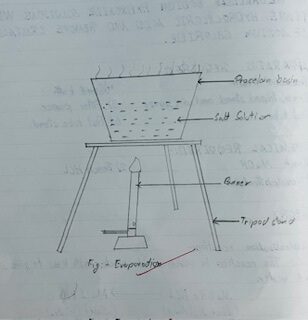
PROCEDURE
About /test tube, dil. NaOH, solution was taken in porcelain basin and 1-2 drops of phenolphthalein indicator was added. The solution was turned into pint.
Dropwise dil.HCL solution was added with the help of dropper by stirring the solution just colorless. The neutral solution was evaporated and the crystals of sodium chloride were removed.
RESULT
The pure crystal of chloride can be obtained by the neutralization reaction of bench Na OH with bench HCL.
PRECAUTION
i) The mixture should be completely neutral.
ii) All the apparatus should be handled carefully.
iii) Separate glass tubes should be used for base bottles.
