APPARATUS REQUIRED:
i) Burettes (2)
ii) Test tubes (8)
iii) Pipettes
iv) UV-visible spectrophotometer
CHEMICALS REQUIRED:
i) Potassium permanganate (KMnO4)
ii) Water (H2O)
THEORY:
Lambert’s Beer’s law states that the absorbance of a substance in a solution is directly proportional to its concentration and the path length of the light passing through the solution.
Mathematically, it is expressed as,
A= ϵLC
where, A = absorbance
ϵ = Molar absorptivity
L= path length
C = concentration
Absorbance of a known concentration of substance is measured by a uv-visible spectrophotometer of a specific wavelength. The principle of uv-visible spectrophotometer is based on the interaction between light and matter. By plotting a graph of absorbance against concentration,we can verify Lambert’s beer’s law and determine the concentration of unknown solution.
PROCEDURE:
i) Prepared a 0.0005 M stock solution of potassium permanganate by dissolving 0.0079 gm of KMnO4 in 100ml of water.
ii) Eight test tubes were taken.
iii) 1 test tube (0.00005 M solution): 1 ml of KMnO4 stock solution and 9 ml of water were added to the test tube by pipette.
iv) 2nd test tube (0.0001 M solution); To 2 ml of KMnO4 stock solution, 8 ml of water was added.
v) 3rd test tube (0.00015 M solution); To 3 ml of KMnO4 stock solution, 7 ml of water was added.
vi) 4th test tube (0.0002 M solution ): To 4 ml of kMnO4 stock solution, 6 ml of water was added.
vii) 5th test tube (0.00025M solution); To 5 ml of KMnO4 stock solution, 5 ml of water was added.
viii) 6th test tube (XM solution); A solution of unknown concentration was made by mixing unknown volume of water and volume was made upto 10ml.
ix) The solutions should be thoroughly mixed to ensure the KMnO4 was completely dissolved in the water.
x) 7th test tube : used water as a blank.
xi) 8th test tube : used pure kMnO4 stock solution as preference.
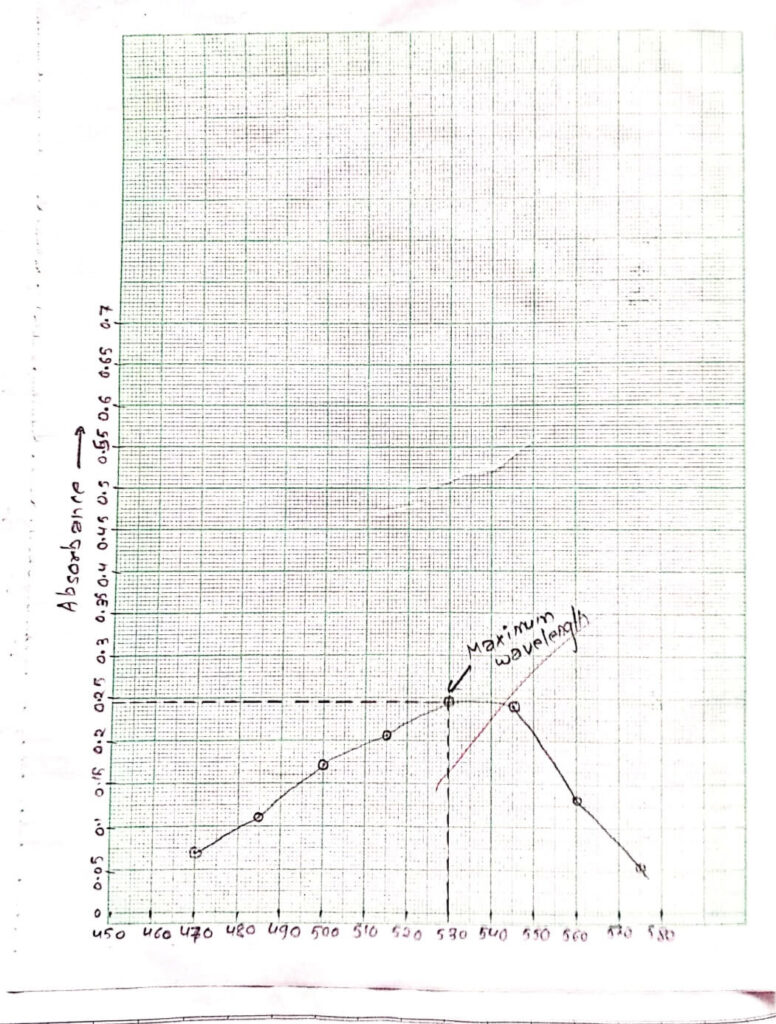
xii)The maximum wavelength of KMnO4 was determined using a spectrum or graph plotting in graph paper. This wavelength was used for maximum sensitivity in absorbance measurement.
xiii) A graph with absorbance on x-axis and concentration on Y-axis was plotted. It was ensured that the graph shows a linear relationship.
xiv) The concn of unknown solution was determined by comparing its absorbance value to the graph.
OBSERVATION:
Graph was plotted for maximum wavelength between wavelength and absorbance.
| Wavelength(nm) | Absorbance |
| 470 | 0.070 |
| 485 | 0.119 |
| 500 | 0.173 |
| 515 | 0.209 |
| 530 | 0.249 |
| 545 | 0.240 |
| 560 | 0.133 |
| 575 | 0.108 |
Now, at 530 nm kMnO4 have maximum absorbance 0.249
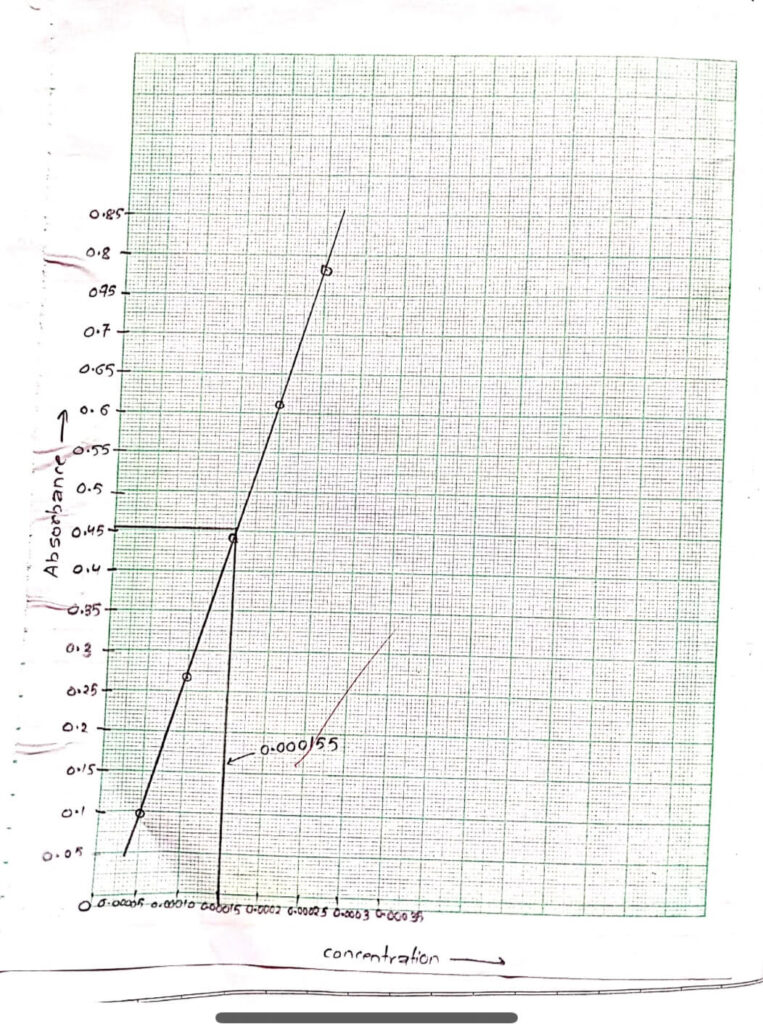
Graph was plotted between concentration and absorbance.
| Concentration (M) | Absorbance |
| 0.00005 | 0.098 |
| 0.00010 | 0.267 |
| 0.00015 | 0.442 |
| 0.00020 | 0.613 |
| 0.00025 | 0.782 |
| Unknown | 0.457 |
From the graph, the unknown concentration of KMnO4 was 0.000165 M.
RESULT:
This experiment was valid for Lambert’s Beer’s law and the unknown concentration at KMnO4 was found to be 0.000165 M.
More From Physical Pharmacy | 1st Year |
- Verify Lambert’s Beer’s Law & Determine KMnO4 Concentration
- To Determine Sedimentation Volume of Suspensions with Various Agents
- Sedimentation Volume: Effect of Bentonite Concentrations
- TO DETERMINE THE SURFACE TENSION OF A LIQUID (LIQUID PARAFFIN) USING STALAGMOMETER BY DROP WEIGHT METHOD AT ROOM TEMPERATURE
- Determine Solubility of Barium Chloride in Water
- Determine Bulk Density of Starch Powder: Formula & Method
- TO DETERMINE THE FLOW PROPERTY OF SUCROSE BY ANGLE OF REPOSE BY FIXED FUNNEL METHOD.
- Surface Tension of Liquid Paraffin: Drop Number Method
- Determine Specific Gravity of Glycerol Using Pycnometer
- Determine Benzene Viscosity Room Temp By OSTWALD’S U-TUBE VISCOMETER.