APPARATUS REQUIRED:
a) Test tube
b) Test tube holder
c) Beaker
d) Tripod Stand
e) Filter paper.
CHEMICALS REQUIRED:
a) Aniline
b) Conc. HCI
c) NaNO2
d) β-Naphthol
THEORY:
Phenyl azo-β-Naphthol is a common example of azodye. It is prepared by coupling reactions in the laboratory. It is prepared by action of sodium nitrite and conC.HCI with alkaline β-Naphthol ice cold temperature.
NaNO2 + HCL ————-> HNO3 +NaCl
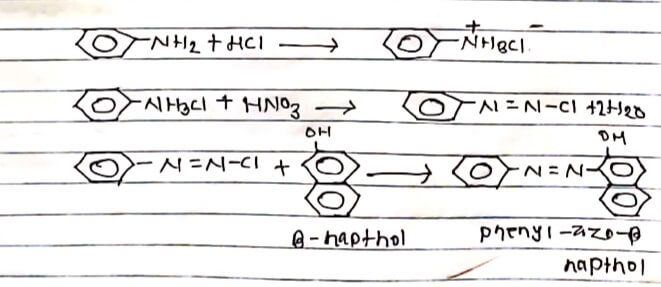

PROCEDULE:
5 ml of aniline was dissolved in a mixture of conc. HCl and 10 ml of water. The solution was cooled in Ice cold water and then 1 ml of sodium nitrate was added in solution dropwise, with continuous Shuking and controlling the temperature below 5°C.
In another flask, 5 gm of β-Naphthol was dissolved in 19 of NaOH in 15 ml of water and then cooled in the cold water. The 1st mixture was added to the secondary prepared mixture drop by drop with continuous timing. The produce thus formed was filtered and then washed with cold water and dried. The filtrate thus obtained was phenyl -azo-β-Naphthol.
RESULT:
Thus, phenyl -azo-β-Naphthol was obtained from the aniline which works as dye.
CONCLUSION:
Hence, dyes can be prepared in the laboratory by diazotization reaction.
PRECAUTIONS:
1) Apparatus should be handled carefully.
2) The Solution should be cooled in ice water.
3) Reagents should be used in the proper amount.
