METHOD 1
THEORY:
The presence of moisture or water vapour in air can be readily shown by the behavior of a deliquescent substance when exposed to the atmosphere. A deliquescent substance is the one which absorbs moisture from the air and ultimately passes into the liquid state. Fused calcium chloride and caustic soda are deliquescent substances.
APPARATUS :
1) Watch glasses
CHEMICALS:
1) Dry caustic soda
2) Fused anhydrous calcium chloride.
PROCEDURE:
i) Put a piece of dry caustic soda (NaOH) and a piece of fused calcium chloride on two separate watch glasses, and leave them exposed to the air.
ii) Look for the traces of moisture after 20-30 minutes.
METHOD II
THEORY:
The presence of moisture in the air can also be shown by the effect of cooling the air sufficiently in contact with a cooled surface.
APPARATUS :
(1) Conical flask
2) Clamps
CHEMICALS:
1) Sodium chloride
2) Ice
PROCEDURE:
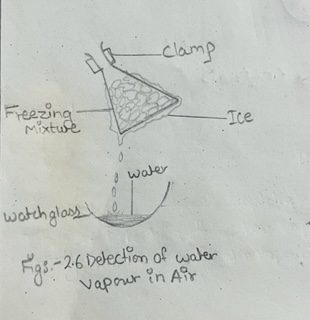
i) Mix crushed ice and common salt in the approximate ratio of 10:1. This is called ‘freezing mixture’ which has a temperature lower than 0°C.
ii) Fill a dry conical flask with the mixture, and allow it to stand, being suspended in clamp, for about half an hour as shown in fig. 2.6.
iii) Ice will be seen to form on the outside of the flask.
iv) Scrape off the ice on a clock glass.
v) After some time, the ice on the clock glass melts into liquid. Test the liquid with a little anhydrous copper sulphate when it turns blue. It shows that the liquid formed is water.