THEORY:
The presence of carbon dioxide in air can be shown by its action on lime water. Carbon dioxide turns lime water milky.
Ca(OH)₂ + CO₂ ———–> CaCO3 + H₂O
milky
However, if excess of CO₂ is passed, the milky white suspension disappears giving a clear solution of soluble calcium bicarbonate.
CaCO3 + H₂O + CO₂ ————–> Ca(HCO3)₂
APPARATUS:
1) Test tubes
2) Glass tubes
3) Bottles (W.M.) or woulfe’s bottles
4) Suction pump
CHEMICALS:
Lime water
PROCEDURE:
METHOD I
i) Take a little (about 5 ml) of lime water in a clean test tube, and blow in exhaled air through it by means of a glass tube the ends of which are already rounded.
ii) Note that the lime water turns milky first, and becomes clear again if blowing is continued for a pretty long time. It shows the presence of carbon dioxide in exhaled air.
METHOD II
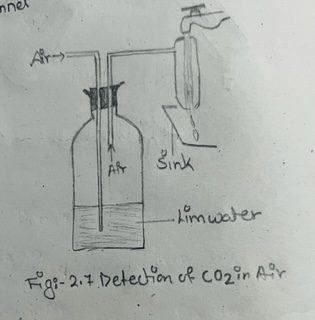
i) Assemble the apparatus as shown in fig. 2.7.
ii) When water runs down through the pump, a stream of air is continuously sucked in through the lime water in the bottle.
iii) Bubble the air through the lime water in the bottle for about twenty minutes when the solution gradually turns milky or turbid.
This experiment clearly indicates the presence of carbon dioxide in open air as well.