THEORY:
Chromatography is a simple technique of separating micro-quantities of substances in a mixture. It was first discovered by a Russian scientist Tswett in 1903. It can be done in various ways. One of the simple ways of doing it is to use paper chromatography.
The separation of mixture by paper chromatography is based on the fact that different substances are adsorbed to different extents by the cellulose fibers of the paper. The components which are more firmly adsorbed on paper are carried away less by a moving solvent, whereas those which are less firmly adsorbed are carried away further across the paper. Thus the different components become separated in different coloured bands.
The Rf value of a component is defined as
Rf = distance traveled by the component from the original line / distance traveled by the solvent from the original line
APPARATUS:
1) 500 ml beaker
2) Filter paper [Whatman)
3) Plastic paper clips
4) Fine capillary tube
CHEMICALS:
1) Black ink or universal indicator
PROCEDURE:
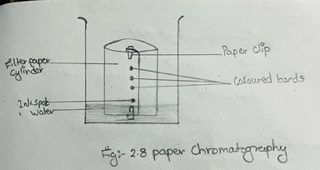
i) Cut a filter paper five inches square and draw a line with a pencil about 3 cm above from one edge.
ii) With the help of the capillary tube put a spot of ink or universal indicator at the middle of the pencil line. Let it air dry. Put another drop on the same spot and dry again.
iii) Now, roll the paper up into the shape of a cylinder. Fix the two ends with plastic clips.
iv) Place the paper cylinder in a large beaker containing water + methanol such that the pencil line and the spot is about 2 cm above the level of the solvent.
v) Leave it undisturbed for about half an hour.
vi) Take out the filter paper when you will see that the components of the mixture spot are separated into different coloured bands.
vii) Mark the distance traveled by the solvent front with a pencil.
viii) Let the paper dry in the air. Also put pencil marks at the centers of different coloured bands.
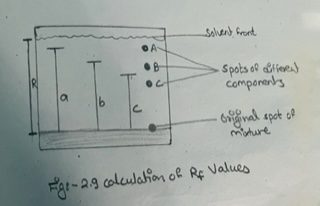
ix) Measure the various distances traveled by the different components from the original line and also the distance traveled by the solvent from the original line and then calculate the Rf values of different components (see fig. 2.9)
Rf value of component A =a / R
Rf value of component B = b / R
And so on.
Different substances have different Rf values.