THEORY:
Pure water is a weak electrolyte. It ionises very little. Hence it is not a good conductor of electricity. But if it is acidulated with dil H₂SO4, it becomes a good conductor. It is because sulphuric acid, being a strong electrolyte, ionises completely to give plenty H+ ions and SO4 — ions in the solution.
(i) H₂SO4 → 2H+ + SO4 — (complete ionisation)
(ii) 4H₂O ——–right left harpoons—————–> 4H++ 4(OH)– (feeble ionisation)
So we have three types of ions namely H+ ions, (OH)- ions and SO4– ions in the aqueous solution. On passing electric current from the external battery, the cations (H+ ions) move towards the cathode, and the anions (OH ions and SO4– ions) move towards the anode.
The H+ ions are discharged at the cathode to form H₂ gas, but at anode. only (OH) ions are discharged (not SO4 — ions) to give O₂ gas. It is because (OH) ions have lower discharge potential than SO4 — ions.
Hence,
Cathode reaction:
4H+ + 4e(from cathode)————>4H = 2H2 ……….. (iii)
Anode reaction:
4(OH)– – 4e(to anode) ————->4(OH) = 2H₂O + O₂…………(iv)
on adding (ii), (iii) and (iv)
4H₂O ————>2H₂O + 2H₂ + O₂
2H₂O ———-> 2H₂ + O₂
As H+ ions and (OH) are gradually consumed, the equilibrium of process (ii) will be disturbed, and it will be shifted to the right. Hence water ionises more and more to provide the required number of H+ions and (OH)– ions in the solution. The amount of H₂SO4 added initially remains constant. The hydrogen and oxygen gasses are thus seen to result essentially from the decomposition of water only.
It will be seen that for every one volume of oxygen gas collected at the anode compartment, two volumes of hydrogen will be collected at the cathode compartment.
APPARATUS:
1) 10 V battery
2) Hoffmann’s voltameter
3) Ammeter
4) Connecting wires.
Alternatively
1) 10 V battery
2) U-tube with side arms
3) Ammeter
4) Platinum electrodes
5) Rubber tubing
6) Delivery tubes
7) Rubber corks
8) Pyrex test tubes
9) Beakers
10) Key
11) Connecting wires.
CHEMICALS:
1) Dil. sulphuric acid.
A) PROCEDURE :
(If Hoffman’s voltameter is not available)
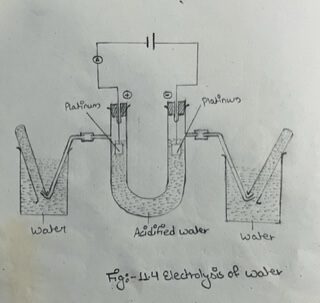
(a) Arrange the apparatus as shown in fig. III, and fill the U-tube to a level about 2 cm below the side arms with dil. H₂SO4 solution.
(b) Connect the electrodes to an ammeter and a 10 volt battery through a key.
(c) Note the formation of gasses at both the electrodes.
(d) Allow 2 or 3 minutes for the air to be swept out of the apparatus, and then only collect the gasses in the test tubes.
(e) Compare the volumes of the gasses collected.
(f) Test the anode gas with a glowing splint, and the cathode gas with a lighted taper.
CONCLUSION:
Water is composed of hydrogen and oxygen in the ratio 2:1 by volume.
(B) PROCEDURE:
(If Hoffman’s voltameter is available)
In case, Hoffman’s voltameter is available, the electrolysis may be directly carried out by taking the dil. H₂SO4 in the voltameter. The results seen will be the same.
Note:
(i) Either platinum electrodes or graphite electrodes may be used in this experiment.
(ii) This experiment may be used by the teacher for demonstration in the laboratory.
