THEORY:
A. Gaseous Diffusion :
Diffusion of gasses means the intermixing of gasses through each other in spite of gravitation.
PROCEDURE:
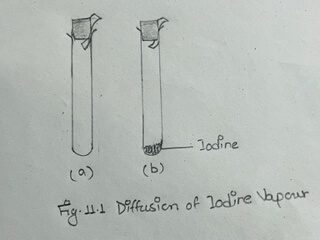
i) Take two dry test tubes in a test tube rack. Hold a piece of starch paper in the mouth of one test tube with the help of a cork (Fig. 11. 1a)
Do the same in another test tube after introducing a small iodine crystal in it. (Fig. 11.1b)
Wait for a while and see that a blue color appears on the starch paper in the second test tube containing iodine crystal.
This is obviously due to the gradual diffusion of iodine vapour through air in the test tube.
ii. Take two clean and dry gas jars. Put one drop of conc. HCI at the bottom of one of them and a drop of liquor ammonia at the bottom of the other. Invert the jar containing ammonia over the other containing conc, HCI mouth to mouth. White dense fumes of NH4CI will be formed due to the diffusion of the two vapours through each other. lodine
B. Dispersion of A Soluble Solid Through Solvent:
Dispersion of a soluble solid through the solvent means the diffusion of solute particles through the solvent molecules from the region of high concentration to the region of low concentration till a homogeneous mixture is formed.
PROCEDURE :
i) Take 10 ml of a 2% solution of KMnO4 in a beaker (use a measuring cylinder). Add 90 ml of water and stir.
ii) Take 10 ml of the solution from (a) and put it into another beaker, and add 90 ml of water and stir.
iii) Similarly, dilute the solution from (b) ten times in the third beaker. See that the slight pink colour of KMnO4 can still be seen in the third beaker.
iv) Calculate the weight of KMnO4 in one drop of the final solution in gm and mole.
[Assume 1 ml = 20 drops]