APPARATUS REQUIRED:
1) Conical flask
2) Beaker
3) Filter paper
4) Thistle funnel
5) Testtube
6) Water bath
CHEMICAL REQUIRED:
1) Dimethyl aniline
2) Sodium carbonate
3) Sodium nitrite
4) Sulphanilic acid
5) Conc.HCL
6) Glacial acetic acid
THEORY:
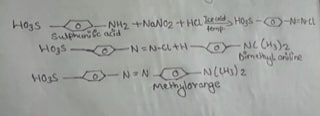
Methyl orange is a light orange coloured solid soluble in alcohol. It is prepared as above by the diazotization. of sulphanilic acid followed by coupling with dimethyl aniline in presence of acid.
PROCEDURE:
0.5 gm sodium carbonate was dissolved in 20ml, water with 2gm sulphanilic acid. The solution was cooled in ice cold water & added a mixture of 1gm NaNO₂ & 2 ml of water. Then 2 ml of conc. HCL was added dropwise by maintaining temperature between 0-40°C, followed by a solution of 1 ml dimethyl aniline in 1 ml of glacial acetic acid. The solution was filtered and washed with water and dried, finally recrystallized in hot water & detected its melting point.
RESULT:
Appearance: light orange crystal
M.P. : above 3000c.
CONCLUSION:
Hence, preparation of Methyl orange from sulphanilic acid was done in the laboratory.
PRECAUTION:
1) All the apparatus should be dried & cleaned before experiment.
2) Temperature must be maintained during the experiment as stated.
