APPARATUS REQUIRED:
1) Conical flask,
2) Beaker,
3) Test tube,
4) Filter paper etc.
CHEMICAL REQUIRED:
5ml aniline, 7ml Benzal chloride, 40 ml NaOH
THEORY:
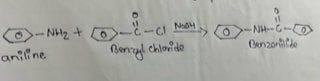
Benzaldehyde is prepared in the laboratory by Benzylation of aniline with Benzylchloride in presence of sodium hydroxide.Replacement of H atom of -NH₂ group of aniline by benzoyl
group takes places. Hence the process is known as Benzoylation.
PROCEDURE:
In a 250ml conical flask, take 5 ml aniline, 40 ml of NaOH & 7 ml of Benzoyl chloride. The solution was shaken till benzoyl chloride disappeared. Then the solution was filtered for 10-15 min & washed with the cold water. Finally the ppt was recrystallized from cold alcohol.
RESULT:
Appearance: White crystal solid
Yield: 9 am
Melting paint: 102°C
CONCLUSION:
Hence, the preparation of benzanilide by the benzoylation of aniline was done in the laboratory,
PRECAUTION:
1) All the apparatus should be clean & dry.
2) Shake well for 10-15 minutes.
3) Recrystalize should be done by using cold alcohol.
